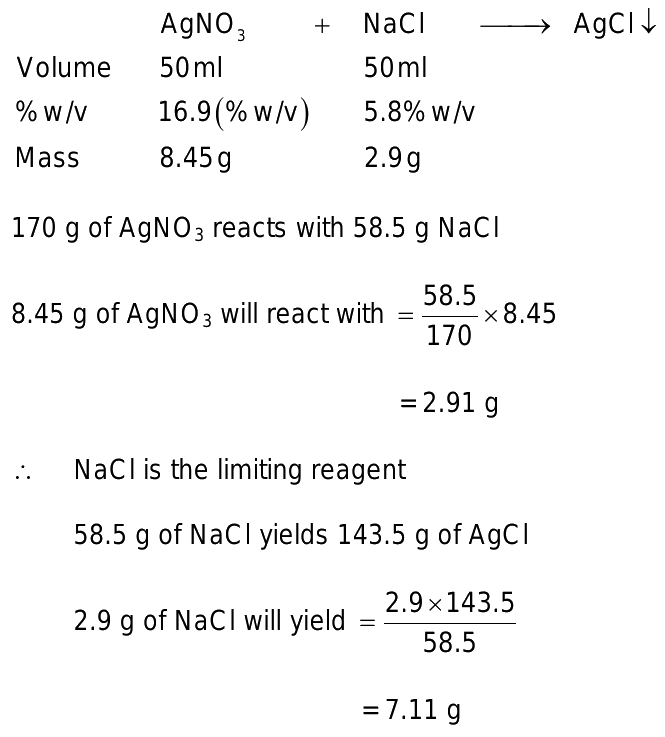
What is the mass of precipitate formed
We endeavor to keep you informed and help you choose the right Career path. When you look back in lifethis app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
What is the mass of precipitate formed
Q: A What is the mass, in…. Q: Many metal ions are precipitated from a solution by the sulfide ion. As an example, consider…. Q: Barium hydroxide is mixed with lithium phosphate at room temperature. If Q: Many metal ions are precipitated from solution by the sulfide ion. As an example, consider treating…. Q: A student weighs out a 1. Q: 10 mL of a 30 M sodium phosphate solution….
Proteins purification and separation can be performed by precipitation in changing the nature of the solvent or the value of its relative permittivity e.
Questions » Accounting » Auditing » What mass of precipitate in g is formed when Questions Courses. What mass of precipitate in g is formed when May 15 AM. Subhash P answered on May 17,
Chemistry Glossary Definition of Precipitate. In chemistry, to precipitate is to form an insoluble compound either by reacting two salts or by changing the temperature to affect the solubility of the compound. Precipitation may indicate a chemical reaction has occurred, but it may also occur if a solute concentration exceeds its solubility. Precipitation is preceded by an event called nucleation, which is when small insoluble particles aggregate with each other or else form an interface with a surface, such as the wall of a container or a seed crystal. The terminology can seem a bit confusing. Here's how it works: forming a solid from a solution is called precipitation.
What is the mass of precipitate formed
A chemist combines mL of a 0. How many grams of precipitate form? By solubility rules, is soluble in water and is not. Our new reaction is:. Now we perform the same calculation beginning with :. The limiting reagent is and this reaction produces
Nuworks
Enthalpy of Formation. Naming Cyclic Alkanes. Relating Solubilities to Solubility Constants The solubility by which we usually mean the molar solubility of a solid is expressed as the concentration of the "dissolved solid" in a saturated solution. Extensive Properties. Introductory Chemistry: A Foundation. Clearly the reagents are present in molar ratio. Longo; J. HBr is a strong electrolyte. Equilibrium Constant Calculations. As an example, consider…. Cancel out all spectator ions those that appear as ions on both sides of the equation. A must visit Q: Calculate the mass, in grams, of potassium iodide that must be added to a mL volumetric flask in…. For example, syrup and honey are oversaturated sugar solutions, containing other substances such as citric acids. Lattice Energy.
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
Intro to Crystal Field Theory. Constant-Pressure Calorimetry. A: The question is based on the concept of Reaction Stoichiometry. Gibbs Free Energy. Just comparing the ion product Q s with the solubility product K s p. Crystal Field Theory Summary. Spam Answer drives traffic to external sites for promotional or commercial purposes. The ionic equation is after balancing :. How many Laboratory Materials. Equilibrium Constant K. For oversatureated solutions, Q sp is greater than K sp. The ions replace each other based on their charges as either a cation or an anion.

You are not right.
Who knows it.