What is the correct name for n2o4
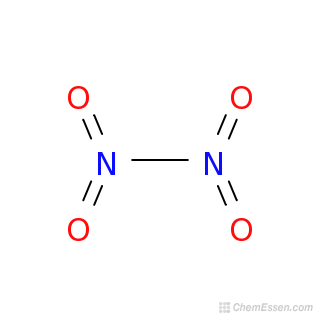
The Dinitrogen tetroxide molecule contains a total of 6 atom s. There are 2 Nitrogen atom s and 4 Oxygen atom s, what is the correct name for n2o4. A chemical formula of Dinitrogen tetroxide can therefore be written as:. The chemical formula of Dinitrogen tetroxide shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural information, which is different from the empirical formula which provides the numerical proportions of atoms of each type.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons.
What is the correct name for n2o4
.
Germany Israel United States Japan.
.
Explore more content. Cite Download 4. Since as early as the s, dinitrogen tetroxide N2O4 has been regarded as a promising oxidizer in rocket propulsion systems. In more recent times, its predecessor, mixed oxides of nitrogen MON , remains a top contender among oxidizers, due to its unique characteristics such as low freezing temperature and compatibility with common spacecraft materials. Today, these N2O4-based oxidizers are the preferred choice in many upper stages, launch escape systems, reaction control systems, liquid apogee engines, and in-space primary propulsion systems. N2O4-based oxidizers are a key factor in rocket propulsion, and thoroughly understanding their history, development, characteristics, synthesis, and composition analysis are crucial for space exploration today and into the future. To fully understand and predict the physical properties of a MON sample, it is important to measure and quantify its chemical composition.
What is the correct name for n2o4
Some families name a son usually the first born after his father. So, it is somewhat common to meet a John Smith, Jr. Countries with long histories of royalty take the naming even further. One line of kings named Henry goes up to Henry the Eighth not the nicest guy in the world—he had six wives and two of them met untimely ends. The use of numbering for names adds clarity to a system—it's easily discernible which Henry is being spoken of. Inorganic chemical compounds can be broadly classified into two groups: ionic compounds and molecular compounds.
Dog sitting hiring
It can provide a standard way to encode the molecular information of Dinitrogen tetroxide to facilitate the search for the compound information in databases and on the web. Journal of the American Chemical Society. Toggle limited content width. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. The tendency of N 2 O 4 to reversibly break into NO 2 has led to research into its use in advanced power generation systems as a so-called dissociating gas. Various anhydrous transition metal nitrate complexes can be prepared from N 2 O 4 and base metal. GHS labelling :. Retrieved Wikimedia Commons has media related to Dinitrogen tetroxide. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4. Hazard statements. Tools Tools.
It is a useful reagent in chemical synthesis.
The law of conservation of mass dictates that the quantity of each element given in the chemical formula does not change in a chemical reaction. Various anhydrous transition metal nitrate complexes can be prepared from N 2 O 4 and base metal. In early , research on the usability of dinitrogen tetroxide as an oxidizing agent for rocket fuel was conducted by German scientists, although the Germans only used it to a very limited extent as an additive for S-Stoff fuming nitric acid. Phosphorus tetroxide Arsenic tetroxide Antimony tetroxide. Additionally, NTO is often used with the addition of a small percentage of nitric oxide , which inhibits stress-corrosion cracking of titanium alloys, and in this form, propellant-grade NTO is referred to as mixed oxides of nitrogen MON. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. N 2 O 4 was the main component of the "nitrin" working fluid in the decommissioned PamirD portable nuclear reactor which operated from to Signal word. Its molar mass is This was due to a switch accidentally left in the wrong position, which allowed the attitude control thrusters to fire after the cabin fresh air intake was opened, allowing NTO fumes to enter the cabin. Archived PDF from the original on 7 May Nitric acid is manufactured on a large scale via N 2 O 4.

It not meant it
I join. So happens. Let's discuss this question.
I think, that you are mistaken. Let's discuss it. Write to me in PM, we will communicate.