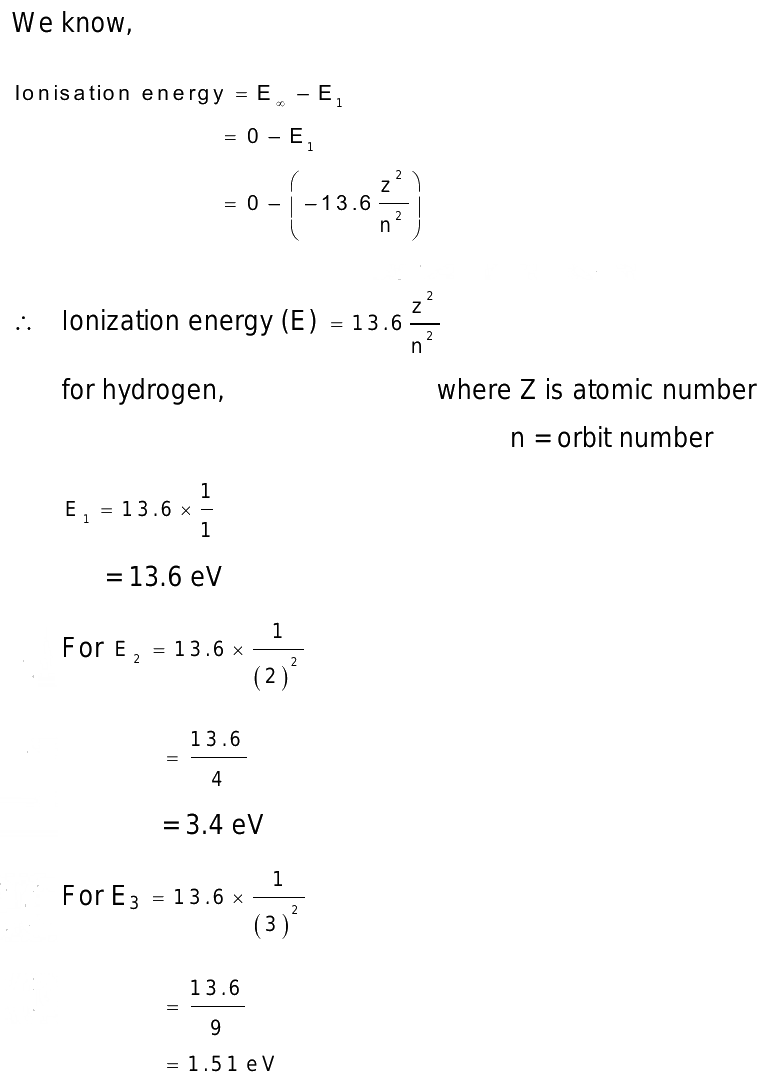
The ionization energy of hydrogen atom is 13.6
The ionisation potential of hydrogen atom is The ionization potential of hydrogen atom is When an electron in the hydrogen atom in ground state absorb a photon of energy
Isotopes: The elements which have the same atomic number but different mass numbers are called isotopes. Last updated on May 25, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
The ionization energy of hydrogen atom is 13.6
Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams. Insurance Exams. Nursing Exams.
Patna Civil Court Peon.
Ionization potential of hydrogen atom is Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy The spectral lines emitted by hydrogen atoms according to Bohr's theory will be. The ionization potential of H-atom is The H-atoms in ground state are excited by mono chromatic radiations of photon energy
In physics and chemistry , ionization energy IE is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom , positive ion , or molecule. Roughly speaking, the closer the outermost electrons are to the nucleus of the atom , the higher the atom's ionization energy. In physics, ionization energy is usually expressed in electronvolts eV or joules J. Comparison of ionization energies of atoms in the periodic table reveals two periodic trends which follow the rules of Coulombic attraction : [4]. The latter trend results from the outer electron shell being progressively farther from the nucleus, with the addition of one inner shell per row as one moves down the column. For example, the first three ionization energies are defined as follows:.
The ionization energy of hydrogen atom is 13.6
Ionization energy, in simple terms, can be described as a measure of the difficulty in removing an electron from an atom or ion or the tendency of an atom or ion to surrender an electron. The loss of electrons usually happens in the ground state of the chemical species. Alternatively, we can also state that ionization or ionization energy is the measure of strength attractive forces by which an electron is held in a place. In more technical terms, we can describe ionization energy as the minimum energy that an electron in a gaseous atom or ion has to absorb to come out of the influence of the nucleus. It is also sometimes referred to as ionization potential and is usually an endothermic process. What we can deduce further is that ionization energy gives us an idea of the reactivity of chemical compounds.
Pornboil com
AOC Material Assistant. Bihar Secondary Teacher. Three photons coming from excited atoms hydrogen sample are picked up SECL Operator. MP Forest Guard. RRB Office Assistant. Patna Civil Court Group C. UP Police Head Operator. KSP Constable. Banking Exams.
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy. This is the energy required to remove an electron, in this case, from a molecule:.
The ionization energy of hydrogen atom is BHU Staff Nurse. RRB Junior Translator. Banking Exams. Rajasthan Informatics Assistant. Patna High Court Personal Assistant. JEE Main. Telangana Police SI. MP Civil Judge. Airforce Group Y. Odisha Amin.

Yes, really.
It is already far not exception