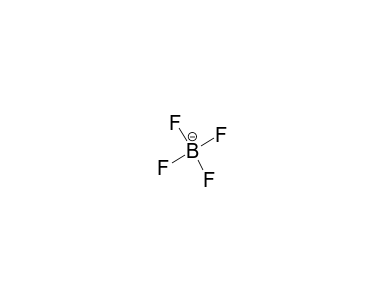
Tetrafluoroborate
Don't have a profile? Potassium tetrafluoroborate is used as an active filler in the preparation of tetrafluoroborate abrasives. It is also used for extraction, refining and processing of metals in the chemical industry, tetrafluoroborate.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Tetrafluoroborate
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience. With a formula weight of Moreover, in other cases of ostensibly "cationic" complexes, the fluorine atom in fact acts as a bridging ligand between boron and the cationic center. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and cesium. Imidazolium and formamidinium salts, ionic liquids and precursors to stable carbenes , are often isolated as tetrafluoroborates. Contents move to sidebar hide. Article Talk.
Tetrafluoroborate density Not determined, tetrafluoroborate. Additonal code used was developed at NIST: jcamp-dx. Additional technical, research and safety SDS information is available.
Silver tetrafluoroborate is an inorganic compound with the chemical formula AgBF 4. It is a white solid that dissolves in polar organic solvents as well as water. Silver tetrafluoroborate is prepared by the reaction between boron trifluoride and silver oxide in the presence of benzene. In the inorganic and organometallic chemistry laboratory, silver tetrafluoroborate, sometimes referred to "silver BF-4", is a useful reagent. In dichloromethane, silver tetrafluoroborate is a moderately strong oxidant. The abstraction of the halide is driven by the precipitation of the appropriate silver halide. This inorganic compound —related article is a stub.
With an accout for my. It arises by the reaction of fluoride salts with the Lewis acid BF 3 or by treatment of tetrafluoroboric acid with base. Safety considerations, however, overshadow this inconvenience. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and caesium. Fluoroborate salts are often associated with highly reactive compounds. Some examples:. Categories: Fluorides Non-coordinating anions.
Tetrafluoroborate
Unlike other strong acids like H 2 SO 4 or HClO 4 , the pure solvent free tetrafluoroboric acid more precisely, pure hydrogen tetrafluoroborate H[BF 4 ] does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The solvent can be any suitable Lewis base.
Severance based on a book
Danger of explosion: Not determined. It is one of three metals that occur as a liquid at room temperature, the others being mercury and gallium. Avoid transfer into the environment. The selection of suitable gloves not only depends on the material, but also on quality. Pipette Tips and Racks. DE please activate JavaScript. Some examples:. The fluorine atom has a covalent radius of 64 pm and its Van der Waals radius is pm. All Nanomaterials Quantum Dots. In the inorganic and organometallic chemistry laboratory, silver tetrafluoroborate, sometimes referred to "silver BF-4", is a useful reagent. GHS07 Skin Irrit.
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience.
Your browser is not current. Linear Formula: CsBF 4. Prop 65 - Developmental toxicity, male Substance is not listed. The word Cesium originates from the Latin word "caesius," meaning "sky blue," which refers to the vibrant blue lines in its spectrum. Bioaccumulative potential No further relevant information available. Control parameters Components with limit values that require monitoring at the workplace: The product does not contain any relevant quantities of materials with critical values that have to be monitored at the workplace. Contents move to sidebar hide. May cause respiratory irritation. Information about protection against explosions and fires: The product is not flammable Conditions for safe storage, including any incompatibilities Storage Requirements to be met by storerooms and receptacles: No special requirements. Solubility in water. Safety considerations, however, overshadow this inconvenience. Further, it is used in the production of fluxing agents for brazing and soldering. Relative density Not determined. Cesium is a member of the alkali group of metals. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and cesium.

It is remarkable, this rather valuable message