So2 o2 so3 balanced
Skip to main content.
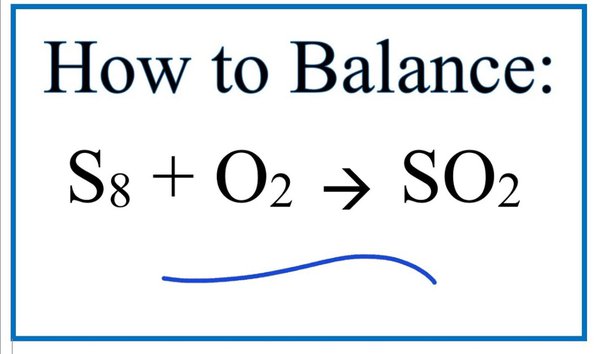
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26, Explanation: You follow a systematic procedure to balance the equation. Another useful procedure is to start with what looks like the most complicated formula. Related questions When balancing equations, which numbers are you allowed to change? How do I get the chemical equation of aniline to phenylisocyanide? What is a balanced equation? Why do chemical equations need to be balanced?
Nuclear Chemistry 2h 34m. Reaction Mechanism. Aluminum and hydrochloric acid react to form aluminum
.
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left.
So2 o2 so3 balanced
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
Cornelio reyna
Osmotic Pressure. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Standard Reduction Potentials. Constant-Pressure Calorimetry. This method uses algebraic equations to find the correct coefficients. Periodic Trend: Cumulative. Bronsted-Lowry Acids and Bases. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Periodic Trend: Electron Affinity. Constant-Volume Calorimetry. Solubility Rules. Titrations: Weak Acid-Strong Base. Explanation: You follow a systematic procedure to balance the equation. Noble Gas Compounds. Electron Configurations of Transition Metals: Exceptions.
The coefficients show the number of particles atoms or molecules , and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms.
Intro to Henry's Law. Sodium phosphate and calcium chloride react to form MO Theory: Bond Order. The Ideal Gas Law Applications. Balancing Chemical Equations Practice Problems. Each half-reaction is balanced separately and then combined. Acid-Base Indicators. Weak Titrate-Strong Titrant Curves. Rate of Radioactive Decay. Hydrohalogenation Reactions. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. There are already "4 O" atoms on the left. Neutron to Proton Ratio. Skeletal Formula.

I apologise, but, in my opinion, you are not right. I am assured. I can prove it. Write to me in PM.
I am sorry, that I interrupt you, would like to offer other decision.
I apologise, but, in my opinion, you commit an error. Write to me in PM.