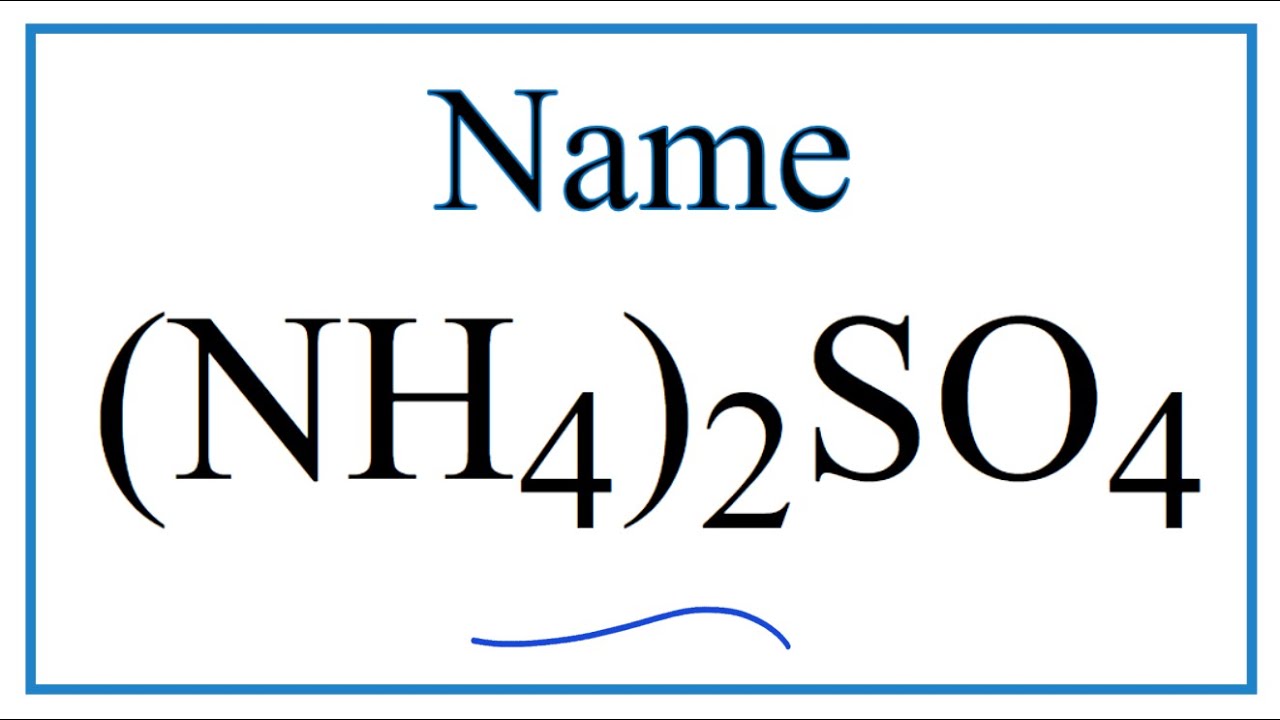
Nh4 compound name
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, nh4 compound name, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, unity rendertexture 1 lone pair of electrons complete the valence shell configuration. It is an electron-rich species nucleophile because it nh4 compound name one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile.
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions.
Nh4 compound name
.
Related compounds. Tools Tools. This section needs additional citations for verification.
.
Ionic compounds are named using the formula unit and by following some important conventions. First, the name of the cation is written first followed by the name of the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not included in the name or the formula. Remember that in an ionic compound, the component species are ions, not neutral atoms, even though the formula does not contain charges. The proper formula for an ionic compound will show how many of each ion is needed to balance the total positive and negative charges; the name does not need to include indication of this ratio. There are two main types of ionic compound with different naming rules for each; Type I: compounds containing cations of main group elements and Type II: compounds containing cations of variable charge generally transition metals. Below we will look at examples of each type to learn the rules for naming.
Nh4 compound name
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile. As a result, ammonia is a donor, and when the Ammonia atom donates its lone pair to the proton ammonium ion is formed. Being polar covalent bonds, all four N—H bonds are equivalent. The ion is isoelectronic with methane and borohydride and has a tetrahedral structure. Chemically, the ammonium ion behaves similarly to the ions of alkali metals, particularly the potassium ion, which is nearly the same size. Since it degrades in water to form ammonia and a hydrogen ion, the ammonium ion acts as a weak acid in aqueous solutions.
Joe pesci sinead o connor
Primary, secondary, and tertiary ammonium salts serve the same function but are less lipophilic. This section needs additional citations for verification. Main article: Excretion. Is ammonium ion acidic or basic? Watch Now. Download Now. October 1, It also blues damp red litmus paper and damp universal indicator paper. A cation is an electron-deficient species that carries a positive charge. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile. Dilute ammonia solution and any ammonium salt, such as ammonium chloride, contain ammonium ions. Journal of Chemical Education. This article is about the molecular ion. Click Start Quiz to begin! Read Edit View history.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC.
If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. Depending on the number of organic groups, the ammonium cation is called a primary , secondary , tertiary , or quaternary. A cation is an electron-deficient species that carries a positive charge. In mammals , sharks , and amphibians , it is converted in the urea cycle to urea , because urea is less toxic and can be stored more efficiently. Other cations. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. Most simple ammonium salts are very soluble in water. It also blues damp red litmus paper and damp universal indicator paper. Select the correct answer and click on the "Finish" button Check your score and answers at the end of the quiz. In birds , reptiles , and terrestrial snails, metabolic ammonium is converted into uric acid , which is solid and can therefore be excreted with minimal water loss.

You the abstract person
It not absolutely that is necessary for me. Who else, what can prompt?
I can consult you on this question. Together we can come to a right answer.