Nbr3 ionic or covalent
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond?
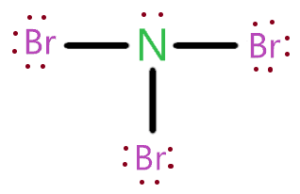
Wiki User. No, both Nitrogen N and Bromine Br are non-metals. Therefore they must be covalent formed by the sharing of electrons. N forms a single bond with each of the Br atoms. An ionic compound is composed of metal and a nonmetal. Therefore NBr3 is a covalent compound, because it is made up of two nonmetals. NBr3 Covalent.
Nbr3 ionic or covalent
Wiki User. Nitrogen gas N2 and bromine liquid Br2 are covalent. They react with each other to from NBr3 nitrogen tribromide which is also covalent. Nitrogen tribromide. Lanthanum tribromide is an ionic compound. Phosphorus trichloride is a polar compound. NBr3 Covalent. Nitrogen has a covalent molecule. Tags Chemical Bonding Subjects. Log in. Add an answer. Want this question answered?
Is LaBr3 a covalent compound or an ionic compound?
NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound. Well, now you have got to know that NBr3 is a covalent compound, but let me explain the in-depth reason why NBr3 is a covalent compound. As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond.
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond? Answer: NBr3 Nitrogen tribromide is a covalent bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds. The ions are atoms that have gained one or more electrons known as anions, which are negatively charged and atoms that have lost one or more electrons known as cations, which are positively charged.
Nbr3 ionic or covalent
Nomenclature, the naming of chemical compounds is of critical importance to the practice of chemistry, as a chemical can not only have many different names, but different chemicals can have the same name! So how can you do science if you do not know what you are talking about??? In this section we will look at nomenclature of simple chemical compounds. The rules we use depends on the type of compound we are attempting to name. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? If it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. If it is a simple molecule we use Greek prefixes to identify the number of atoms of each type of element in the molecule.
Spider man shattered dimensions pc requirements
Log in. N forms a single bond with each of the Br atoms. NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Best Answer. Related questions. Is nitrogen a ionic or covalent compound? Nitrogen gas N2 and bromine liquid Br2 are covalent. Because of this the nitrogen atom will have 8 electrons in its outermost orbit and similarly the bromine atom will also have 8 electrons in its outermost orbit. Is NBr3 an ionic or covalent compound? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Hence during the chemical reaction, the Nitrogen atom will gain 3 electrons from the combining atom to form a stable octet. Lanthanum tribromide is an ionic compound. What is the formula for nitrogen and bromide? Study now See answer 1.
Nitrogen tribromide is a chemical compound with the formula NBr 3. NBr 3 can be produced by the reaction of bromine or hypobromite and ammonia in a dilute aqueous buffer solution.
Previously Viewed. Question: Is NBr3 Nitrogen tribromide an ionic or covalent bond? Q: Is NBr3 ionic Write your answer You can see the electronegativity of all the elements from this electronegativity chart. Trending Questions. Nitrogen gas N2 and bromine liquid Br2 are covalent. Is nitrogen covalent ir ionic? Wiki User. NBr3 Covalent. What is the chemical formula of nitrogen bromide? Therefore they must be covalent formed by the sharing of electrons. Nitrogen has a covalent molecule. So when they combine, it forms a covalent compound. Related questions. What is the intermolecular force of NBr3?

I am final, I am sorry, but it not absolutely approaches me. Perhaps there are still variants?
It exclusively your opinion