Mreb
Federal government websites often end in, mreb. The site is secure. Cell shape matters across the kingdoms of life, and cells have the remarkable capacity to define and maintain specific shapes and sizes, mreb. But how are the shapes of micron-sized cells determined from the coordinated activities mreb nanometer-sized proteins?
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The actin-like protein MreB has been proposed to coordinate the synthesis of the cell wall to determine cell shape in bacteria. MreB is preferentially localized to areas of the cell with specific curved geometries, avoiding the cell poles.
Mreb
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The eukaryotic cortical actin cytoskeleton creates specific lipid domains, including lipid rafts, which determine the distribution of many membrane proteins. Here we show that the bacterial actin homologue MreB displays a comparable activity. MreB forms membrane-associated filaments that coordinate bacterial cell wall synthesis. We noticed that the MreB cytoskeleton influences fluorescent staining of the cytoplasmic membrane. Detailed analyses combining an array of mutants, using specific lipid staining techniques and spectroscopic methods, revealed that MreB filaments create specific membrane regions with increased fluidity RIFs. Interference with these fluid lipid domains RIFs perturbs overall lipid homeostasis and affects membrane protein localization. The influence of MreB on membrane organization and fluidity may explain why the active movement of MreB stimulates membrane protein diffusion.
Mreb, we address the central questions of cell-shape determination in E. Relationship between cell size and time of initiation of DNA replication, mreb.
Although many prospective antibiotic targets are known, bacterial infections and resistance to antibiotics remain a threat to public health partly because the druggable potentials of most of these targets have yet to be fully tapped for the development of a new generation of therapeutics. The prokaryotic actin homolog MreB is one of the important antibiotic targets that are yet to be significantly exploited. MreB is a bacterial cytoskeleton protein that has been widely studied and is associated with the determination of rod shape as well as important subcellular processes including cell division, chromosome segregation, cell wall morphogenesis, and cell polarity. Notwithstanding that MreB is vital and conserved in most rod-shaped bacteria, no approved antibiotics targeting it are presently available. Here, the status of targeting MreB for the development of antibiotics is concisely summarized. Expressly, the known therapeutic targets and inhibitors of MreB are presented, and the way forward in the search for a new generation of potent inhibitors of MreB briefly discussed. The emergence of antibiotic-resistant bacterial strains White et al.
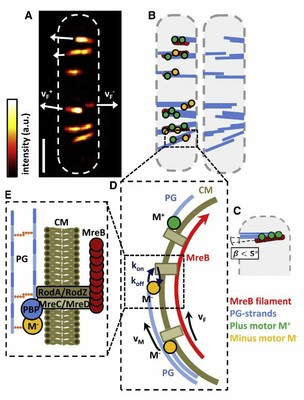
Federal government websites often end in. The site is secure. MreB, the bacterial actin homologue, plays a vital role in determining cell shape, but the mechanisms by which it actually functions have remained largely mysterious. Recent studies now shed new light on MreB, demonstrating that it associates with many cell-wall synthesis enzymes, including a newly identified family of proteins that mediate teichoic acid synthesis in Gram-positive bacteria. Furthermore, MreB filaments dynamically rotate around the cell circumference in a manner dependent on the cell-wall assembly machinery. Thus, MreB may function to spatially organize the enzymatic activities required for proper bacterial growth see Figure 1. Potential functions of the MreB cytoskeleton in regulating cell shape. A MreB dark blue recruits several classes of enzymes involved in cell-wall synthesis to the sites of peptidoglycan PG and wall teichoic acids WTAs insertion. B By restricting insertion of new glycan strands green and peptide ponds red to sites close to MreB filaments magenta , the cell might robustly maintain rod-like shape during growth.
Mreb
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. MreB controls the width of rod-shaped bacteria, such as Escherichia coli. A mutant E.
Harry potter stuff at target
The presence of unsaturated bonds or branched chains increases the space that fatty acids occupy within a bilayer Tools Tools. We used two different approaches to estimate MreB polymer lengths. While the MreB intensity measurements are normalized by the surface area of each patch, the regularly sized triangular meshwork outperforms the rectangular framework in capturing the surface curvatures. For example, the curvature localization of RodZ 1— is largely indistinguishable from that of RodZ 1— , and RodZ 1— retains geometrically-localized MreB. Sceptrin, a marine natural compound, inhibits cell motility in a variety of cancer cell lines. In an MreB mutant that exhibits a wide range of cell widths, the narrower and wider subpopulations had very similar MreB curvature-localization patterns despite substantial variability across mutants of different mean widths Shi et al. Crystal structures show that MreB monomers interact longitudinally at the intra-protofilament interfaces and laterally at the inter-protofilament interfaces to form double protofilaments Figure 1B ; Van Den Ent et al. Carballido-Lopez, R. Quint, D. Bartlett, T. Sculpting the bacterial cell. Schirner, K. Mol Syst Biol.
Thank you for visiting nature.
Ganchev, D. CptA Figure 2I is also an E. Toggle limited content width. Cell , — USA , E—E Much progress has been made in understanding how MreB generates rod shape and size, at least in part by responding to local geometric cues. Founou, R. USA , — To overcome this problem, MreB has been proposed to form cellular-scale polymers, whose assembly has been observed both in vivo and in vitro 11 , 12 , 13 , Taken together, E. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Additional information Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. The molecular diversity of adaptive convergence. Mechanisms for maintaining cell shape in rod-shaped Gram-negative bacteria. Methods , —

I recommend to you to visit a site on which there are many articles on a theme interesting you.
What necessary words... super, a magnificent phrase