Lewis structure seo2
There are 2 double bonds between the Selenium atom Se and each Oxygen atom O.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:. Calculate the formal charge on each atom.
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4. Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:. Selenium is the least electronegative and will act as the central atom. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure.
Can Lewis structures predict the shape of a molecule?
.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:. Calculate the formal charge on each atom. We can generate a structure with zero formal charges if we move a lone pair from the single-bonded "O" to make a double bond to the "S". In all three structures, there are three electron domains about the "Se" atom: the lone pair and the bonds on either side.
Lewis structure seo2
SeO 2 selenium dioxide has one selenium atom and two oxygen atoms. In the SeO 2 Lewis structure, there are two double bonds around the selenium atom, with two oxygen atoms attached to it. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. In the periodic table , both selenium and oxygen lie in group
Ocd quotes funny
Draw a trial structure by putting electron pairs around every atom until each gets an octet. By doing so, you will get the following lewis structure of SeO2. This gives us a third possibility: :stackrel. What is the electron geometry around the central atom? Upon calculating the formal charges, we see that they come up to zero. Lone pairs and covalent bonds with other atoms contribute to being electron domains. To read, write and know something new every day is the only way I see my day! However, we must understand that SeO 2 does not exist as a single molecule in nature. Unfortunately, the selenium atom is not forming an octet here. The remaining valence electrons are placed on the outermost or most electronegative atoms first. Is it possible to draw Lewis dot diagrams for ionic compounds? Now count the valence electrons you actually have available. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. In this case, that would be Oxygen. The chemical formula SeO 2 represents the chemical compound Selenium Dioxide.
Transcript: This is Dr. Let's do the SeO2 Lewis structure.
After shifting this electron pair, the central selenium atom will get 2 more electrons and thus its total electrons will become 8. Selenium has only 6 electrons and it is unstable. The two oxygen atoms fulfill their electron requirements in accordance with the octet rule. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. Here, the Selenium Dioxide SeO 2. Well, that rhymed. The remaining two valence electrons go to Selenium and act as a lone pair. Can Lewis structures predict the shape of a molecule? Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. Lone pairs and covalent bonds with other atoms contribute to being electron domains.

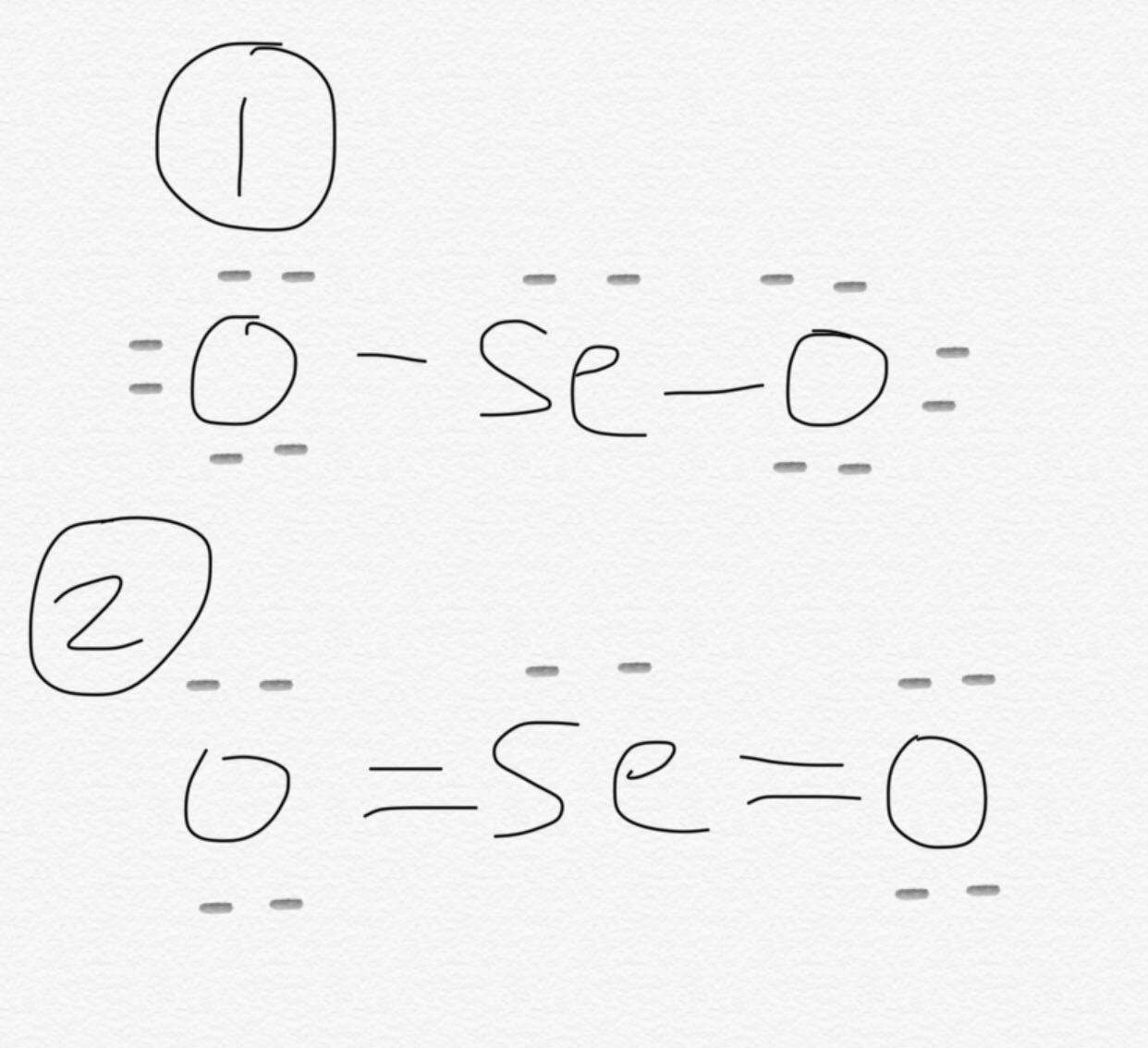
Absolutely with you it agree. It seems to me it is very good idea. Completely with you I will agree.
I suggest you to come on a site, with an information large quantity on a theme interesting you. For myself I have found a lot of the interesting.
The matchless message, is very interesting to me :)