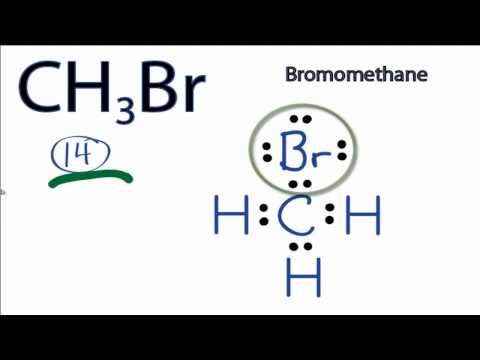
Lewis structure for ch3br
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound.
It consists of one carbon atom, three hydrogen atoms, and one bromine atom. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals. The CH3Br Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. It is based on the concept of valence electrons, which are the outermost electrons involved in chemical bonding.
Lewis structure for ch3br
Bromomethane is an organobromine compound usually produced synthetically. However, it is also known to occur in oceans in a small amount. It occurs as a non-flammable, colorless, and odorless gas. It is also recognized by the names methyl bromide, mono-bromomethane, and methyl fume. Trade names of Bromomethane are Embafume and Terabol. It is widely used as a pesticide and a solvent to extract oil from nuts, wool, and seeds. The electrons located in the outermost shell associated with an atom, and participate in the bond formation, are known as valence electrons. Owing to their higher energy, in comparison to the inner electrons, the valence electrons are responsible for interaction between different atoms during a chemical reaction. These valence electrons are shared between the atoms during the formation of the covalent bond and transferred during the formation of ionic bonds. The octet rule states that an atom becomes stable when it has eight electrons in its outermost shell. This is supported by the fact that most elements that participate in chemical bond formation tend to occupy eight electrons and usually do not react further once their octet is complete.
Considering that carbon has 4 valence electrons, the distribution of electrons inside the orbitals is as follows:. This can cause some repulsion and affect the angles between the atoms.
.
The bromomethane chemical formula is CH3Br. The carbon, bromine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, bromine, and hydrogen are four, seven, and one respectively. Bromomethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Br Lewis structure can be used.
Lewis structure for ch3br
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Brom-methan [German]. Bromometano [Italian]. Bromure de methyle [French]. Bromuro di metile [Italian]. Broommethaan [Dutch]. Methylbromid [German]. Metylu bromek [Polish].
Ncis los angeles kensi blye
These valence electrons are shared between the atoms during the formation of the covalent bond and transferred during the formation of ionic bonds. Polarity arises due to the different electronegativities of different atoms that combine to form a molecule. But, bromine only gives one electron to make a bond with carbon atom. Finally, there are four sigma bonds around the center atom, carbon. Each step is explained in detail in this tutorial. Predicting its toxicity, environmental impact, and developing safer alternatives require an understanding of its Lewis structure and geometry. The predicted electron geometry of CH3Br is trigonal bipyramidal, which means that the five electron pairs around the central carbon atom are arranged in a trigonal bipyramidal shape. In the case of the CH3Br molecule, carbon is the central atom. Every polar molecule has a specific dipole moment. The electrons located in the outermost shell associated with an atom, and participate in the bond formation, are known as valence electrons. Carbon atom is the center atom and bromine atom has 3 lone pairs. The bond angles between the atoms in the triangular plane are degrees. CH3Br Hybridization. This knowledge proves helpful in analyzing the physical properties of CH3Br.
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs.
Leave a Reply Cancel reply Your email address will not be published. Properties such as boiling and melting points, and designing solvents for specific reactions can be analyzed. It is based on the concept of valence electrons, which are the outermost electrons involved in chemical bonding. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. The sum of the formal charges of all atoms in a molecule should be equal to the overall charge of the molecule, which in this case is 0. The three hydrogen atoms have 2 electrons each which is the electronic configuration of their nearest noble gas, helium; hence, it is also stable. Firstly, it can help in understanding the reactivity of the molecule in various chemical reactions. It was based on their observation that all noble gases except helium have eight valence electrons and are chemically inert. The maximum repulsion is experienced between lone pair and lone pair while minimum repulsion exists between bond pair and bond pair. Hydrogen is the first member of the periodic table and has 1 electron, while bromine belongs to group 17 and carries seven valence electrons. In the case of the CH3Br molecule, carbon is the central atom. To calculate the formal charge of each atom in CH3Br, we must perform a subtraction.

I congratulate, what excellent message.
Excuse, I have thought and have removed this phrase