Lewis dot structure questions class 11
This action cannot be undone.
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. Which of the following correctly represents the Lewis dot structure of the CO molecule:. BF 3 is a planar and an electron deficient compound.
Lewis dot structure questions class 11
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Write the Lewis structures for each of these molecules. Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, Cl 2 CO. Write the Lewis structures for carbon tetrachloride and phosgene. The arrangement of atoms in several biologically important molecules is given here.
Think one of the answers above is wrong? Which of the following correctly represents the Lewis dot structure of the CO molecule:.
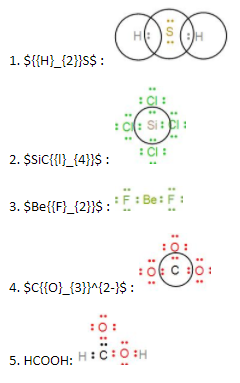
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule.
A Lewis structure is a picture of a molecule that shows the covalent bonds and pairs of free electrons. The octet rule is the basis for Lewis structures. Lewis structures are useful for describing chemical bonds but have some flaws. Let us study Lewis dot structures in detail. Lewis Dot Structures. A Lewis structure is a way to show the shape of a molecule. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. By drawing a Lewis dot structure, you can find the lone electron pairs in molecules, which helps you figure out how chemical bonds form. Molecules with covalent bonds and compounds held together by ions can have Lewis structures. Covalent bonds work because electrons are shared.
Lewis dot structure questions class 11
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. In Lewis dot structures each dot represents an electron. A pair of dots between chemical symbols for atoms represents a bond.
Crop top suit set
H 3 PO 3 can be represented by structures 1 and 2 shown below. A Lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms. Chemistry All. Select Question Set:. Low ionization enthalpy, Low electron gain enthalpy, Low lattice energy. Do not add any more atoms. Click Here to view all bookmarked questions of the chapter. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Ammonia has the molecular formula NH 3. The steps that must be followed while drawing a Lewis structure are listed below. The Lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom. Subtopic: Ionic Bond. To determine the stability of atoms. Subtopic: M.
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars.
SF 6 ii. Previous Year. How do they differ? The outermost central level of energy-containing electrons is called the level of valence and includes electrons of valence. Download Now. Problem Cyclopentane — What will be the formula and electron dot structure of this element? What Is Sublimation. Previous Doubts. Adelaide Clark, Oregon Institute of Technology. Put your understanding of this concept to test by answering a few MCQs. Past Year - MCQs. SbCl 5 2. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.

I apologise, but, in my opinion, you are mistaken. I suggest it to discuss. Write to me in PM.
In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.