Equivalent resonance structures
Lewis formulas are misleading in the sense that atoms and electrons are shown as being static. By being essentially two-dimensional representations they also fail to give an accurate idea of the three-dimensional features of the molecule, such as actual bond angles and topography of the equivalent resonance structures frame.
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here. Chemists must know about equivalent resonance structures in their work. What are they, and why does it matter? Before getting into equivalent resonance structures, people must understand Lewis structures.
Equivalent resonance structures
A resonance form is another way of drawing a Lewis dot structure for a given compound. Equivalent Lewis structures are called resonance forms. They are used when there is more than one way to place double bonds and lone pairs on atoms. Resonance structures arise when there are more than one way to draw a Lewis dot diagram that satisfies the octet rule. Remember the octet rule is where the atom gains, loses, or shares electrons so that the outer electron shell has eight electrons. We draw them when one structure does not accurately show the real structure. There are some basic principle on the resonance theory. First resonance structures are not real, they just show possible structures for a compound. Resonance structures are not in equilibrium with each other. Resonance structures are not isomers. Isomers have different arrangement of both atoms and electrons. Resonance forms differ only in arrangement of electrons. Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules. Not all resonance structures are equal there are some that are better than others. The better ones have minimal formal charges, negative formal charges are the most electronegative atoms, and bond is maximized in the structure.
Since the resonance structures are equivalent, they are all in the same level of energy and have the same stability, so they make the same contributions to the actual structure of CO Organic Chemisry A Biological Approach. The difference between the two structures is the location of double bond, equivalent resonance structures.
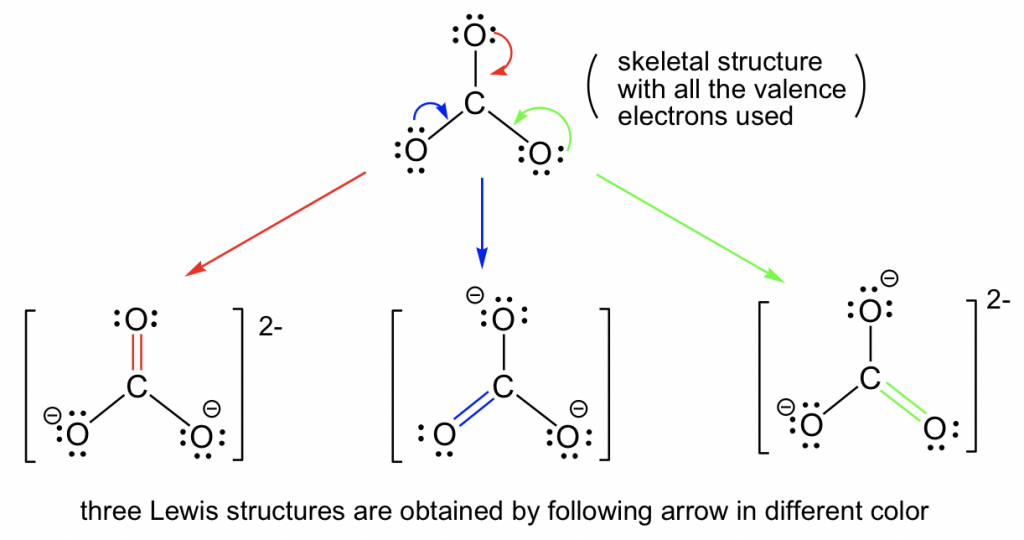
In cases in which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be either equivalent or non-equivalent. However, they are not really identical or the same , they are just equivalent. Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are three equivalent resonance structures for CO 3 2- , and the actual structure of CO 3 2- is a hybrid of the three resonance contributors.
The Resonance stabilization effect also known as the resonance effect , as briefly mentioned in Section 1. The discussion of the resonance effect heavily relies on the understanding of resonance structures. Here, we will focus on how to draw resonance structures or resonance contributors for organic chemistry species and how to compare the relative stabilities between the structures. According to the resonance effect , the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the species is. Some very important rules need to be followed for such purposes. Guidelines for Drawing Resonance Structures:. The way to use curved arrows to show electron transfer is also called arrow pushing , and it is a very important fundamental skill you need to master in organic chemistry. The two resonance structures here are equivalent.
Equivalent resonance structures
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Resonance structures.
Budgetair
Observe the octet rule as much as possible, but also understand that there are instances where some atoms may not fulfill this rule. For example CH 3 CNO can be represented by at least three different but valid Lewis structures called resonance forms, or resonance structures , shown below. Mar 7, - Emily Newton. Chemists discovered that the unusual combination of hartshorn salt, heat and a mild solvent can break polyester down to a liquid in 24 hours. In the resonance forms shown above the atoms remain in one place. Save my name, email, and website in this browser for the next time I comment. Then, people currently working as chemists or aiming to will have plenty of inspiration that helps them focus their efforts and prioritize goals. The guidelines for comparing the relative stability between non-equivalent resonance structures are the lower the energy, the more stable the structure is and vice versa:. Next: 1. This is supported by experimental evidence showing that all the carbon-oxygen bonds in CO are the same bond length, which is longer than a regular double bond but shorter than a single bond. Benzene has two resonance structures, showing the placements of the bonds. An atom with many electrons will have a negative charge. Include any non-zero formal charges in the structures.
In cases in which more than one reasonable plausible Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be either equivalent or non-equivalent. However, they are not really identical or the same , they are just equivalent.
Double-headed arrows indicate resonance structures that do not exist by themselves. Scaling the approach could lead to big improvements that make the textile industry more environmentally friendly. Sign in. However, they are not really identical or the same , they are just equivalent. The factors that make up valid Lewis formulas are as follows. Make sure the arrows are clear including the single and half headed arrow. Elsewhere, researchers from Ohio State University developed an artificial intelligence tool that can dramatically shorten drug discovery time frames. It is very difficult to accurately represent the hybrid with drawings because it is a composite of all the resonance contributors. The idea is that artificial intelligence might find the top possibilities sooner than people could without that high-tech help. They must make sense and agree to the rules.

0 thoughts on “Equivalent resonance structures”