Difference between isothermal and adiabatic process class 11
As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work. This article will definitely help to understand the difference between isothermal and adiabatic process.
Isothermal and Adiabatic Processes are two different processes that are related to heat and energy in thermodynamics. Thermodynamics is the branch of physics which deals with the heat transfer and thermal properties of matter. In thermodynamics, the isothermal process is the process that occurs at constant temperature and the exchange of heat and energy takes place between the system and the surroundings. Adiabatic Process in thermodynamics stands for the process where a system is isolated from its surroundings and there is no heat and energy exchange between the system and the surroundings. In this article, we will learn briefly about the isothermal and adiabatic processes, with a major focus on the difference between isothermal and adiabatic processes. The process in which the temperature of a system remains constant is termed as isothermal process. As the temperature of a system remains constant in an isothermal process, it means that there is an exchange of heat between the system and the surroundings.
Difference between isothermal and adiabatic process class 11
An isothermal process is a thermodynamic process occurring at a constant temperature. Adiabatic process means a process that neither allows the heat to transfer inside nor lets the heat out of the system. For example, a reaction that takes place in a Dewar Flask is adiabatic. In this article, we will discuss adiabatic and isothermal, distinguish between isothermal and adiabatic processes, isobaric isochoric isothermal and adiabatic processes, and understand the process of isothermal adiabatic in detail. Now, we will understand the difference between adiabatic and isothermal:. Thermodynamics uses the concepts of the isothermal process, isochoric process, isobaric process, and adiabatic process to explain the behaviour of a thermodynamic system and its relationship to temperature changes. An isothermal process is a process that happens when there are no variations in the temperature of the system, but other parameters volume, pressure regarding the system can be changed accordingly. Adiabatic process describes a process that remains aloof from its surroundings. It is a process in which no heat transfer occurs between a system and its surroundings. Here, the temperature of the system can vary in order to avoid any heat transfer. This indicates that the main difference between isothermal and adiabatic processes is that the isothermal process takes place under constant temperature whereas the adiabatic process occurs under changing temperature. Isothermal Process. Adiabatic Process.
Change of phase of a substance is an example of an isothermal process.
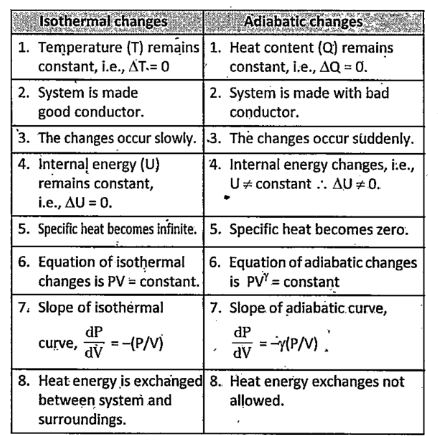
The main difference between an isothermal process and an adiabatic process lies in the heat exchange with the surroundings. The isothermal process, in thermodynamics , is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. It is the process in which the temperature of the system remains constant. The temperature change happens at such a slow rate that thermal equilibrium is maintained. For an isothermal process constant temperature , the ideal gas equation can be given as. That is, the pressure of the gas varies inversely with the volume of the gas. For an ideal gas, the internal energy depends only on the temperature.
The main difference between an isothermal process and an adiabatic process lies in the heat exchange with the surroundings. The isothermal process, in thermodynamics , is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. It is the process in which the temperature of the system remains constant. The temperature change happens at such a slow rate that thermal equilibrium is maintained. For an isothermal process constant temperature , the ideal gas equation can be given as.
Difference between isothermal and adiabatic process class 11
Thermodynamics deals with the two most important concepts of thermal physics, i. The adiabatic process is the one that deals with the transfer of energy between the system and surroundings in the form of work. In an adiabatic process, there is no transfer of any heat or mass between the system and the surroundings.
Laser garage parking assist
One more example is the heat pump, useful to remove the heat from the house. This article is being improved by another user right now. It does not contain the transfer of heat. Work done in an Adiabatic reversible compression is given as. Image will be uploaded soon. The assumption of an adiabatic process is for simplifying the processes and understanding them. Freezing of a liquid: When a liquid freezes, it releases heat to its surroundings. In an adiabatic process, the work done is because of the change in internal energy. Customize your course in 30 seconds Which class are you in? Any transformation in such a process is slow. The process in which the temperature remains constant is called an isothermal process. As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Now learn Live with India's best teachers.
Thermodynamics uses the concepts isothermal process and adiabatic process to explain the behavior of a thermodynamic system and its relation to the temperature changes. Isothermal process is a process that happens under constant temperature, but other parameters regarding the system can be changed accordingly. Adiabatic process describes a process where no heat transfer occurs between a system and its surrounding.
The difference between isothermal and adiabatic processes is that for an adiabatic process, there is no heat flow in and out of the system as the system is well insulated. An adiabatic process can be quickly maintained by doing the process. The graph of isothermal process is shown below. An isobaric process is a process that takes place under constant pressure. Vote for difficulty :. It is a thermodynamic process in which the temperature of a system remains constant with respect to time. Thank you for your valuable feedback! Working in the refrigerator is an isothermal process. As per the difference between the isothermal and adiabatic processes, in an adiabatic process, the energy is transferred only in the form of work done. Example: nozzle, turbine, and compressor. The graph of adiabatic process is shown below. Adiabatic process is a type of thermodynamic process in which where there is no heat transfer. Adiabatic Process. Continue reading to know more about isothermal and adiabatic processes and their differences. Difference between Isothermal and Adiabatic process Parameter Isothermal Adiabatic Definition It is defined as one of the thermodynamic processes which occur at a constant temperature.

0 thoughts on “Difference between isothermal and adiabatic process class 11”