C2h2 2h2
Subsurface chemistry in heterogeneous catalysis plays an important role in tuning catalytic performance. Aiming to unravel the role of subsurface heteroatoms, C 2 H 2 semihydrogenation on c2h2 2h2 series of Pd catalysts doped with subsurface heteroatom H, B, C, c2h2 2h2, N, P, or S was fully investigated by density functional theory DFT calculations together with microkinetic modeling.
By qwerty April 3, in Homework Help. Any help appreciated. Remember that you want everything to be on the correct side of the arrow when you get to the end, like:. The second reaction reaction by 2 and switch the direction which includes changing the sign for the energy. You need to be a member in order to leave a comment. Sign up for a new account in our community. It's easy!
C2h2 2h2
Zhang, K. Jiang, L. Li, Y. Xia, T. Hu, Y. Yang, Y. Cui, B. Li, B. Chen and G. Qian, Chem. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
Acetylene was discovered in by C2h2 2h2 Davywho identified it as a "new carburet of hydrogen". Archived from the original on 20 February Bibcode : Natur.
It is a hydrocarbon and the simplest alkyne. It is unstable in its pure form and thus is usually handled as a solution. As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. Acetylene was discovered in by Edmund Davy , who identified it as a "new carburet of hydrogen". By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide , K 2 C 2 , which reacted with water to release the new gas. He also found that acetylene was formed by sparking electricity through mixed cyanogen and hydrogen gases.
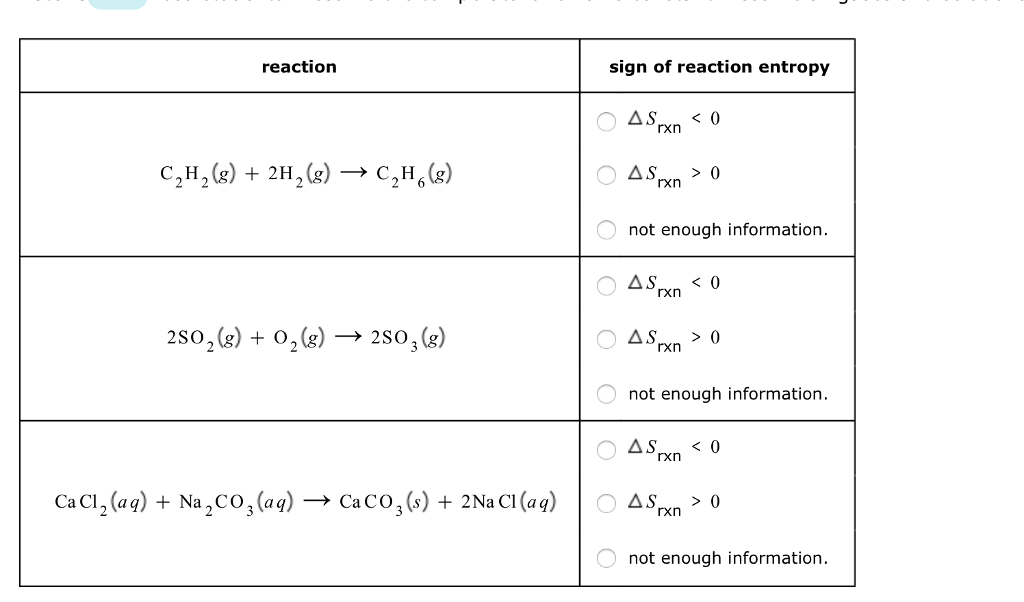
Now that we understand that chemical reactions occur with a simultaneous change in energy, we can apply the concept more broadly. To start, remember that some chemical reactions are rather difficult to perform. For example, consider the combustion of carbon to make carbon monoxide:. In reality, this is extremely difficult to do. Given the opportunity, carbon will react to make another compound, carbon dioxide:. Is there a way around this?
C2h2 2h2
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen. Not only it affects human beings but other living species as well disturbing various natural atmospheric cycles for whom oxygen is an integral component. In light of the same, the recommended airborne exposure limit REL of acetylene is set to ppm Ceiling where an amount greater than this can kill human beings by becoming an asphyxiant gas. With this, it becomes crucial to understand the behavioral chemical properties of acetylene to understand why it behaves in such a specific manner. Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements.
Istanbul atasun optik şubeleri
Rule A By qwerty April 3, in Homework Help. Acetylene cylinders should be used in the upright position to avoid withdrawing acetone during use. In the s, pure acetylene was experimentally used as an inhalation anesthetic. For use in welding and cutting, the working pressures must be controlled by a regulator, since above 15 psi kPa , if subjected to a shockwave caused, for example, by a flashback , acetylene decomposes explosively into hydrogen and carbon. Qian, Chem. Metal Ions in Life Sciences. New York: Marcel Dekker, inc. Bell Canada cable-repair technicians still use portable acetylene-fuelled torch kits as a soldering tool for sealing lead sleeve splices in manholes and in some aerial locations. Similarly, vinylpyrrolidone and vinylcarbazole are produced industrially by vinylation of 2-pyrrolidone and carbazole.
.
In the Pd-B 0. Acetylene has a p K a of 25, acetylene can be deprotonated by a superbase to form an acetylide : [51]. Toggle limited content width. Archived PDF from the original on 11 June Copper acetylide is used as the catalyst. The other two 2p orbitals remain unhybridized. Chemie Ingenieur Technik. Archived from the original on 4 May PEL Permissible. Chemistry of Acetylenes 1st ed. Something went wrong. Inorganic Chemistry 3rd ed. Retrieved 18 February

It agree, the useful message
You are absolutely right. In it something is also idea excellent, I support.
It is remarkable, very amusing opinion