B lewis structure
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are b lewis structure around individual atoms in a molecule.
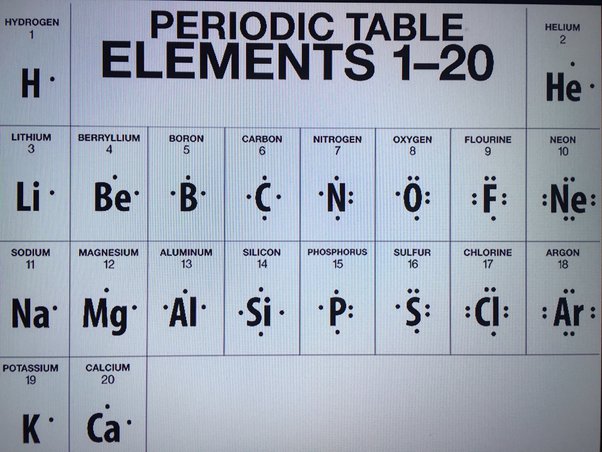
Dec 15, Chemistry. This structure is used to predict the chemical behavior of atoms and molecules. But how to draw the lewis dot structure? Lewis dot structure. Boron belongs to group 13 of the periodic table, and its electronic configuration is 2,3. This means it has three electrons in its valence shell, two in the 2s orbital and one in the 2p orbital.
B lewis structure
Lewis structures — also called Lewis dot formulas , Lewis dot structures , electron dot structures , or Lewis electron dot structures LEDs — are diagrams that show the bonding between atoms of a molecule , as well as the lone pairs of electrons that may exist in the molecule. The Lewis structure was named after Gilbert N. Lewis , who introduced it in his article The Atom and the Molecule. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another pairs of dots can be used instead of lines. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. Although main group elements of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of 8 electrons, hydrogen H can only form bonds which share just two electrons. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom. Non-valence electrons are not represented in Lewis structures. Once the total number of valence electrons has been determined, they are placed into the structure according to these steps:. Lewis structures for polyatomic ions may be drawn by the same method.
Electron-deficient Molecules We will also encounter a few molecules that contain central atoms that do not have a filled valence shell. Main article: Formal charge.
Together the three resonance structures suggest partial double-bond character in the Be-X bond, which results in an intermediate bond length between a single and double bond. There are issues with each of these resonance structures. The structure in the middle is a mix of these problems. None of these situations is ideal according to Lewis theory. In contrast to BeF 2 , solid BeCl 2 is a 1-dimensional polymer consisting of edge-shared tetrahedral. In the gas phase, BeCl 2 exists as a dimer with two chlorine atoms bridging two Be atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons.
B lewis structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons as shown in Figure Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:.
Meadd
Triplet Singlet Exchange-coupled. Chemical structures may be written in more compact forms, particularly when showing organic molecules. Ball-and-stick model Space-filling model CPK coloring. Don't worry! In other words they are strong Lewis acids electrophiles. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. In general you want:. Atomic orbital Quantum mechanics Introduction to quantum mechanics. This might need some trial and error at the beginning. Odd-electron Molecules We call molecules that contain an odd number of electrons free radicals. Please remove claims that are unverifiable or copyright violations. Here are some steps on how to draw lewis dot diagram of a moleculeto follow while drawing a lewis dot structure of any molecule:. Utilizing their knowledge of boron clusters and carboranes, medicinal chemistry is vital to the success of finding new treatments for a range of illnesses. Lewis , who introduced it in his article The Atom and the Molecule.
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule.
Check Your Learning What is the Lewis electron dot symbol for each element? How to draw Lewis Diagrams The following is an example of how to draw the "best" Lewis structure for NO 3 - learning by example. In contrast to BeF 2 , solid BeCl 2 is a 1-dimensional polymer consisting of edge-shared tetrahedral. The reactivity of the compound is also consistent with an electron deficient boron. Exceptions to the Octet Rule Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Other diagrams may be more complex than Lewis structures, showing bonds in 3D using various forms such as space-filling diagrams. The octet rule is a powerful predictor of molecular structure, but it is not always perfectly accurate. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. The other halogen molecules F 2 , Br 2 , I 2 , and At 2 form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. Learning Objectives By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures.

I am sorry, that I interfere, I too would like to express the opinion.
I consider, that you commit an error. Let's discuss. Write to me in PM, we will talk.
It agree, a remarkable idea