Xef2 lewis dot structure
Transcript: Hi, this is Dr. Let's do the XeF2 Lewis structure. Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons.
Xef2 lewis dot structure
This article explains the XeF2 Lewis structure and its characteristics. XeF2 itself is a powerful substance that can both fluorinate and oxidize. Xenon, unlike other noble gases, can react and create different compounds like Xenon tetrafluoride XeF4 and Xenon hexafluoride XeF6. However, the XeF2 Lewis structure is the most stable among them. Determine the total number of valence electrons. Identify the central atom. In XeF2, xenon Xe is the central atom since it is less electronegative than fluorine. Place the central atom and connect it to the surrounding atoms. Distribute the remaining electrons around the atoms. Check if the central atom has an octet. Xenon sits in the fourth energy level and can use the 4d sublevel, which lets it hold more than 8 electrons. Calculate the formal charges for each atom in the molecule. Ensure that they are as close to zero as possible.
See the Big List of Lewis Structures.
.
XeF2 lewis structure is the abbreviation of xenon difluoride. It is one of those rare compounds which involve noble gases despite their strong stability. XeF2 lewis structure and its properties are illustrated in this article. XeF2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Xenon has 8 valence electrons and fluorine has 7 valence electrons. So to form a reliable lewis structure xenon will share its 2 electrons with fluorine forming a single covalent Xe-F bond. This completes the octet stability of fluorine atoms.
Xef2 lewis dot structure
XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Out of these compounds, XeF2 is the most stable one. It is a white.
Top gastroenterologist in richmond va
BrO 3 -. Place the central atom and connect it to the surrounding atoms. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. ClO 4 -. XeF2 is a nonpolar molecule. The final Lewis structure of XeF2 should look like this:. ClO -. So I could try to form a double bond with the Fluorine, but I know Fluorine's very electronegative. BrO 3 -. Let's do the XeF2 Lewis structure. Xenon is the least electronegative. Transcript: Hi, this is Dr. No, xenon Xe in XeF2 does not follow the octet rule. However, the XeF2 Lewis structure is the most stable among them. Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom.
Ensure that they are as close to zero as possible. No, xenon Xe in XeF2 does not follow the octet rule. There are a total of 22 valence electrons in the Lewis structure for XeF2. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. Xenon is in the noble gas group and, as such, can have an expanded valence shell. So we still have two valence electrons left over, we still have a pair left. So I could try to form a double bond with the Fluorine, but I know Fluorine's very electronegative. SO 4 Watch the video and see if you missed any steps or information. Transcript: Hi, this is Dr. Distribute the remaining electrons around the atoms. PO 3 ClO 4 -. Watch the video and see if you missed any steps or information. However, the XeF2 Lewis structure is the most stable among them.

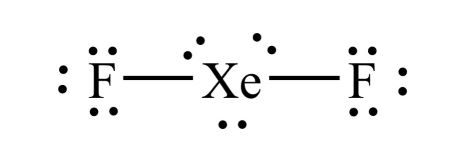
0 thoughts on “Xef2 lewis dot structure”