Why is graphite a good conductor of electricity
Graphite is a good conductor of both electricity and heat. This is due to its molecular structure, which allows electrons to move freely through it. Rather than loosen one sheet of molecules from another, you have to break the covalent bonding the particular way in which atoms are bonded together throughout the whole structure in order to melt the material.
Graphite is a good conductor of heat and electricity because it contains. Therefore, graphite is a good conductor of heat and electricity because it contains free electrons which can conduct heat and electricity. Byju's Answer. Open in App. Conduction of heat and electricity: Graphite is the crystalline allotropes of carbon and it has a layered structure.
Why is graphite a good conductor of electricity
Use app Login. Why is graphite a good conductor of electricity and a good lubricant? Explain with the help of diagram. Open in App. Verified by Toppr. Each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other. Due to this these layers can slip over each other so graphite has a slippery surface. Hence it is used as a lubricant.
Graphite is a great conductor, it is used in electrical cells. Allotropes of Carbon.
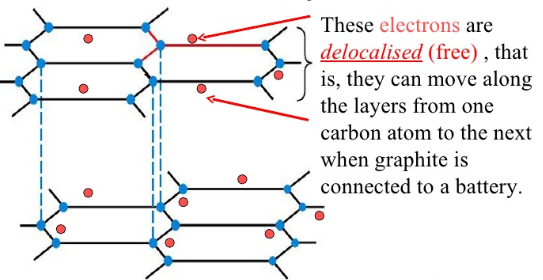
Answer: the very reason why metals do. As she points out, graphite is made from carbon atoms, which have four electrons in their outer shells. Importantly, the layers in graphite are 'aromatic'. Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds see diagram below. This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers.
It is a naturally occurring mineral that is found in metamorphic and igneous rocks. Diamond and Graphite both are mineral of carbon having the same composition but with different chemical structures. Graphite has properties of both metal and non-metal which make it an interesting element. Many students may have a question about whether graphite conducts electricity or not. So, In this article, I will answer this question and cover the surrounding topics too. So, does graphite conduct electricity? Yes, graphite is a very good conductor of electricity because of delocalized electrons.
Why is graphite a good conductor of electricity
Graphite is a fascinating material that has garnered significant attention for its unique properties, particularly its ability to conduct electricity. In this article, we will delve into the reasons why graphite is such a good conductor of electricity, exploring its molecular structure, applications in various industries, and the implications for future technological advancements. Graphite is composed of carbon atoms arranged in a hexagonal lattice, forming layers of interconnected sheets. These sheets are held together by weak van der Waals forces, allowing them to easily slide past each other. This structure gives graphite its characteristic slippery feel and also enables the flow of electrons through the material. In graphite, each carbon atom is bonded to three other carbon atoms, leaving one electron free to move within the material. These delocalized electrons are not confined to a specific bond, allowing them to flow freely through the layers of graphite, carrying electrical current along the way.
Hastings racecourse entries
Synthetic graphite can be produced with varying degrees of porosity—in other words, making the graphite porous so liquid, air or gas can pass through its layers. View More. E-mail: info xrdgraphite. Graphite is a good conductor of both electricity and heat. Also working in academia, Thomas has lectured on the topic of journalism to undergraduate and postgraduate students at The University of Sheffield. Graphite is a good conductor of heat and electricity because it contains :. In other materials, there may not be free cars to make this journey, meaning they're not conductive. This is due to its molecular structure, which allows electrons to move freely through it. Cookies Policy Privacy Policy. Element in the room: chemical elements with mistakes in their names This is why approximately 6, pounds of graphite is used at 14 nuclear reactors across the UK. Both diamonds and graphite have extremely high melting points — both above degrees Celcius. Each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. Graphite is closely related to diamonds.
Answer: the very reason why metals do.
Henan to Impose Off-peak Production i Substances like graphite which have these giant structures have very high melting points. Graphite is a good conductor of heat and electricity because it contains :. Due to this these layers can slip over each other so graphite has a slippery surface. Both diamonds and graphite have extremely high melting points — both above degrees Celcius. This is what makes graphite a soft and slippery material and gives it its self-lubricating properties. However, their structures are significantly different. Graphite made for manufacturing and engineering will have been processed in a way that balances properties such as:. Hence it is used as a lubricant. Writing about everything from cosmology to anthropology, he specialises in the latest psychology, health and neuroscience discoveries.

In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.