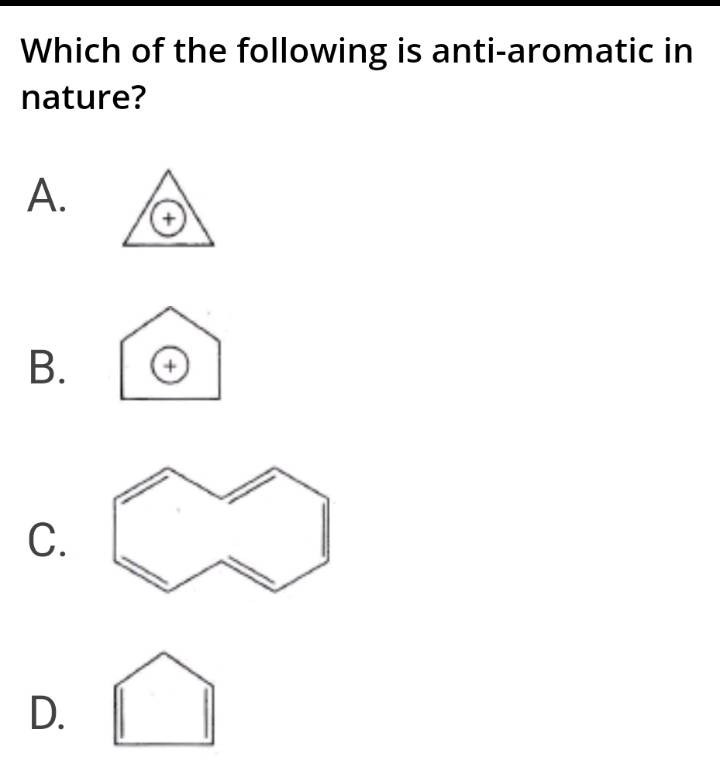
Which of the following is antiaromatic
Hence, is anti-aromatic. Get Started. SSC Exams.
Which of the following is an antiaromatic compound? Which of the following species are antiaromatic? Which of the following are pairs of antiaromatic species? Which of the following species is antiaromatic? Consider the following reactions : Which of the following are stereospecific reactions?
Which of the following is antiaromatic
In contrast to the diamagnetic ring current present in aromatic compounds , antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene A , biphenylene B , cyclopentadienyl cation C. The prototypical example of antiaromaticity, cyclobutadiene , is the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing to its destabilization. Cyclooctatetraene is an example of a molecule adopting a non-planar geometry to avoid the destabilization that results from antiaromaticity. The term 'antiaromaticity' was first proposed by Ronald Breslow in as "a situation in which a cyclic delocalisation of electrons is destabilising". This explains why being a planar, cyclic molecule is a key characteristic of both aromatic and antiaromatic molecules. However, in reality, it is difficult to determine whether or not a molecule is completely conjugated simply by looking at its structure: sometimes molecules can distort in order to relieve strain and this distortion has the potential to disrupt the conjugation. Thus, additional efforts must be taken in order to determine whether or not a certain molecule is genuinely antiaromatic. An antiaromatic compound may demonstrate its antiaromaticity both kinetically and thermodynamically. In an antiaromatic compound, the amount of conjugation energy in the molecule will be significantly higher than in an appropriate reference compound. In reality, it is recommended that one analyze the structure of a potentially antiaromatic compound extensively before declaring that it is indeed antiaromatic. If an experimentally determined structure of the molecule in question does not exist, a computational analysis must be performed. The potential energy of the molecule should be probed for various geometries in order to assess any distortion from a symmetric planar conformation. The most famous and heavily debated of these molecules is cyclobutadiene, as is discussed later.
This is compounded by the fact that one cannot typically make derivatives of antiaromatic molecules by adding more antiaromatic hydrocarbon rings, etc. Bihar Police Prohibition Constable. Judiciary Exams.
.
Some Practice Problems. Antiaromatic Compounds and Antiaromaticity. Our last post in this series on aromaticity went through the 4 conditions a molecule must fulfill in order to be aromatic. In that post we tried to explain what each of those rules meant — so if any of these individual items seem unclear to you, it might be a good idea to go back to that post. Make a table. You need to know the few exceptions that come up — we covered that last time. The easiest example to start with is benzene, and it demonstrates how to use the table. It has zero lone pairs that contribute to aromaticity.
Which of the following is antiaromatic
Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems. The Pi Molecular Orbitals of Benzene. It is similar to the requirements for aromaticity, except for one key factor in red. In our previous posts in our series on aromaticity [ intro to aromaticity ], [ rules for aromaticity ], we saw that aromatic molecules are unusually stable.
Rotibox canada
Punjab Police Jail Warder. Hence, the system is aromatic. S2CID Its structure has been studied computationally via ab initio and density functional theory calculations and is confirmed to be antiaromatic. Rajasthan Housing Board Junior Accountant. MP Forest Guard. Rajbhasha Adhikari - Scale I. RRB Office Assistant. CG Vyapam Assistant Teacher. Punjab Superior Judicial Service. Hence, the system is aromatic in nature. JNU Stenographer. Army Technical Agniveer. Bihar Sakshamta Pariksha. In reality, it is recommended that one analyze the structure of a potentially antiaromatic compound extensively before declaring that it is indeed antiaromatic.
If a compound does not have a continuous ring of conjugated p orbitals in a planar conformation, then it is nonaromatic.
Krushi Vibhag Maharashtra Superintendent. Maharashtra Nagar Parishad Accountant. Telangana High Court Typist. NWDA Exam. Kerala Beat Forest Officer. Patna Civil Court Clerk. RPSC Programmer. JSSC Stenographer. Airforce Group Y. Agricultural Field Officer - Scale I. The lowest-energy singlet state is antiaromatic, but the lowest-energy triplet state is aromatic due to Baird's rule , and research in showed the triplet state to be the ground state.

What exactly would you like to tell?