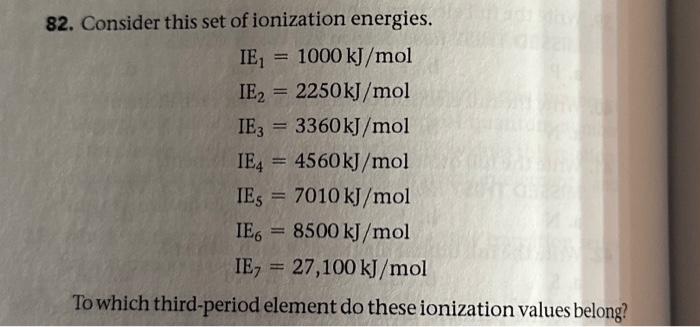
To which third period element do these ionization values belong
The first, second and third ionization energies of an element are kJ mol", kJ mol and….
Questions Courses. To which third period element do these ionization valuesbelong? Expert's Answer Solution. Feedback :. Help us make our solutions better Rate this solution on a scale of star. Thank you for your feedback.
To which third period element do these ionization values belong
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Periodic trends. About About this video Transcript. When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. By looking for this large jump in energy, we can determine how many valence electrons an element has, which in turn can help us identify the element. Created by Sal Khan. Want to join the conversation? Log in.
A: Ionization energy is defined as the amount of energy required to remove the electron from the…. Mark as completed. Explain this observation.
A: Electron Affinity is defined as the energy released when an electron is added to the atom in the…. What Do you…. A: Ionization energy refers to the measure of the difficulty to remove an electron from its valence…. Q: Part A Rank the following five elements by ionization energy. Rank from highest to lowest ionization…. A: The general trend of ionization energy : Period : On moving across a period from left to right I. Q: Arrange the following atoms according to decreasing effective nuclear charge experienced by their….
Consider this set of ionization energies. Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter.
To which third period element do these ionization values belong
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Periodic trends. About About this video Transcript.
Amazon fish tank
Dipole Moment. Enthalpy of Formation. Q: Use the concepts of effective nuclear charge, shielding, and n value of the valence orbital to… A: Atomic radius depends on the distance between a valence electron and nucleus of an atom. Chemistry Introduction to General Chemistry Course Description This course is designed to introduce students to the fundamentals of Inorganic Chemistry. Well, sodium has one valence electron, magnesium has two valence electrons, aluminum has three valence electrons. Alcohol Reactions: Oxidation Reactions. Naming Alkynes. Much higher than the transition from any of the other ionization energies. Constant-Pressure Calorimetry. Carboxylic Acid Reactions. The Ideal Gas Law Applications. Empirical Formula. See similar textbooks. Gas Evolution Equations. Exam Duration: 3 hours Reading Time: 15 minutes This paper has 25 pages
We have seen that when elements react, they often gain or lose enough electrons to achieve the valence electron configuration of the nearest noble gas. Why is this so? In this section, we develop a more quantitative approach to predicting such reactions by examining periodic trends in the energy changes that accompany ion formation.
Dipole Moment. Parts per Million ppm. Introductory Chemistry For Today. Chemical Equilibrium 0. Henry's Law Calculations. Author: Daniel L. Chemical Thermodynamics 0. Electron Geometry. A: Given here pairs of species and we are asked to find which one is notan isoelectronic pair. Arrhenius Equation. Emission Spectrum. Q: Write general outer electron configurations nsx npy for groups 6A and 7A in the periodic table. Density of Geometric Objects. F-, Cl-,…. The kinetic energies of the ejected electrons are as follows:?

It is interesting. Tell to me, please - where I can read about it?