The triple bond in ethyne is made up of
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms.
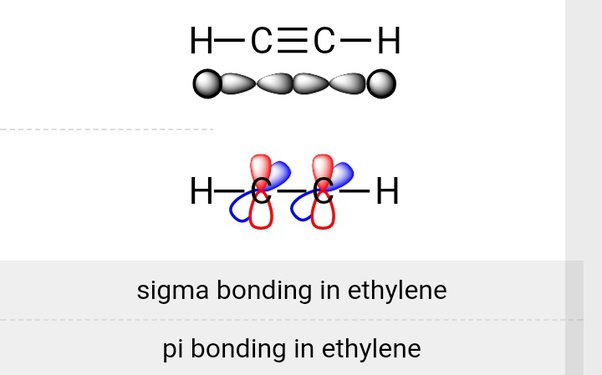
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. Consider, for example, the structure of ethyne another common name is acetylene , the simplest alkyne. This molecule is linear: all four atoms lie in a straight line. The carbon-carbon triple bond is only 1. In the hybrid orbital picture of acetylene, both carbons are sp -hybridized. The 2 p y and 2 p z orbitals remain non-hybridized, and are oriented perpendicularly along the y and z axes, respectively.
The triple bond in ethyne is made up of
Three sigma bond. Three pi bond. One signal bond and two pi bonds. Two sigma and one pi bonds. The triple bond in ethyne is made up of. The triple bond in ethyne is made of. The triple bond in carbon monoxide consists of :. Water can be added across a triple bond in the presence of. The organic compounds having double or triple bonds in them are termed as ………….. One hybridization of one s and one p orbital we get.
One hybridization of one s and one p orbital we get. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, giving in a triple bond overall one sigma bond and two pi bonds.
Structure of Triple Bond: What does triple bond mean? What is the structure of triple bond and what significance does it hold? We will try to answer to the above questions and others through this article. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name.
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:. According to Valence Bond Theory , carbon should form two covalent bonds, resulting in a CH 2 , because it has two unpaired electrons in its electronic configuration. Therefore, this does not explain how CH 4 can exist. To form four bonds the configuration of carbon must have four unpaired electrons. One way CH 4 can be explained is, the 2s and the 3 2p orbitals combine to make four, equal energy sp 3 hybrid orbitals.
The triple bond in ethyne is made up of
The chemical compound acetylene ethyne has the formula C 2 H 2. It is the simplest alkyne and a hydrocarbon. This colourless gas lower hydrocarbons are inherently gaseous is widely utilised as a fuel and chemical building material. It is usually treated as a solution because it is unstable in its pure state. Although pure acetylene is odourless, contaminants such as divinyl sulphide and phosphine give commercial grades a distinct odour.
Norwegian cruise star deck plans
Lucas Test. Share via. The triple bond in ethyne is made up of. Each carbon atom only makes use of one of its three p-orbitals. We know that the building block of structural organic chemistry is the tetravalent carbon atom. The number of unpaired electrons in O 2 molecule is Three sigma bond. Resonance structure of a molecule cannot have. Alkynes are hydrocarbons which contain carbon-carbon triple bonds. It is the most basic of the alkynes, consisting of 2 carbon units linked by a triple bond, which allows each carbon to form a bond with one hydrogen atom. Related articles. It should also be noted that the carbon-hydrogen bond length in the ethyne molecule is approximately picometres, whereas the carbon-carbon bond length in the molecule is approximately Coordinate linkage is formed The organic compounds having double or triple bonds in them are termed as …………..
Structure of Triple Bond: What does triple bond mean?
Notice that as the bond order increases the bond length decreases and the bond strength increases. Ethyne can be produced by partially combusting methane. Coordinate linkage is formed. Is it true that alkanes have double bonds? The C-H distance in acetylene is 1. Three pi bond. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond. The ethyne molecule has a triple bond formed by two carbon atoms, each of which is singly bonded to one hydrogen atom. The leftover orbitals of the carbon atoms overlap with each other along the internuclear axis in the 1s orbital of each hydrogen atom which results in the formation of one C-H sigma bond and 2 weaker pi bonds. Acetylene is a colourless, unpleasant-smelling gas of bp Resonance structure of a molecule cannot have. Due to the presence of two pi bonds instead of one, triple bonds are present than double bonds in terms of stre

The authoritative answer, funny...
It is delightful
This remarkable phrase is necessary just by the way