The quantum number of four electrons are given below
Submitted by James H.
The higher the value of n, the higher the energy of the orbital. Last updated on Nov 2, Get Started. This question was previously asked in. Start Now. Calculation: I. Interested Candidates can submit online applications from 1st November to 30th November
The quantum number of four electrons are given below
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 22, Views: 5, Views: 6, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Structure of Atom. The quantum number of four electrons are given below:. Solving time: 3 mins. Views: 5, students. Updated on: Mar 31, Exam: JEE Mains
Ysu chem F22 lectur… Youngstown State … general chemistry….
Submitted by Anthony M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below.
The principle quantum number , n , describes the energy and distance from the nucleus, and represents the shell. This tells us that the p orbital has 3 possible orientations in space. If you've learned anything about group theory and symmetry in chemistry, for example, you might remember having to deal with various orientations of orbitals. So, we would say that the 2p subshell contains three 2p orbitals shown below. As the name implies, these values describe the spin of each electron in the orbital. Remember that there are only two electrons to every orbital, and that they should have opposite spins think Pauli exclusion principle.
The quantum number of four electrons are given below
There are two fundamental ways of generating light: either heat an object up to be so hot that it glows, or pass an electrical current through a sample of matter usually a gas. Incandescent lights and fluorescent lights generate light via these two methods, respectively. A hot object gives off a continuum of light. This image is known as a continuous spectrum. This image is called a line spectrum. It turns out that every element has its own unique, characteristic line spectrum. Why does the light emitted from an electrically excited gas have only certain colors, while light given off by hot objects has a continuous spectrum? For a long time, it was not well explained.
Pint carlsberg calories
Notes from this class 1 pages. This question was previously asked in. The quantum numbers of six electrons are given below. Click Here to view all bookmarked questions of the chapter. Cod liver oil obtained from fish is rich in:. Add To Playlist Hmmm, doesn't seem like you have any playlists. Class Question 4 Medium. Sign up Login. Snapsolve any problem by taking a picture. The higher the value of n, the higher the energy of the orbital. Add To Playlist Hmmm, doesn't seem like you have any playlists. Was this solution helpful? Sign up Login. Join Numerade as a.
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics.
Hello students for 4 electrons we have given different values of quantum numbers so we need to arrange these electrons in increasing order of their energy so we know that for l equals to 0, 1, 2, 3 we have spdf orbitals now here n is 4 and l is 0 it represents 4s orbital here n is 4 and l is 1 it represents 4p orbital here n is 3 and l is 2 it represents 3d orbital and here n is 3 and l is 1 so it represents 3p orbital now…. Snapsolve any problem by taking a picture. Share Question Copy Link. So we developed a line of study tools to help students learn their way. Join Numerade as a. Submitted by Anthony M. Sign Up Free. Question 2 Medium. Four electrons in an atom have the sets of quantum numbers as given below. Add To Playlist Hmmm, doesn't seem like you have any playlists. Invite sent! What is the maximum number of orbitals that can be identified with the The third quantum number, m, represents the orientation of the electron's orbital in space.

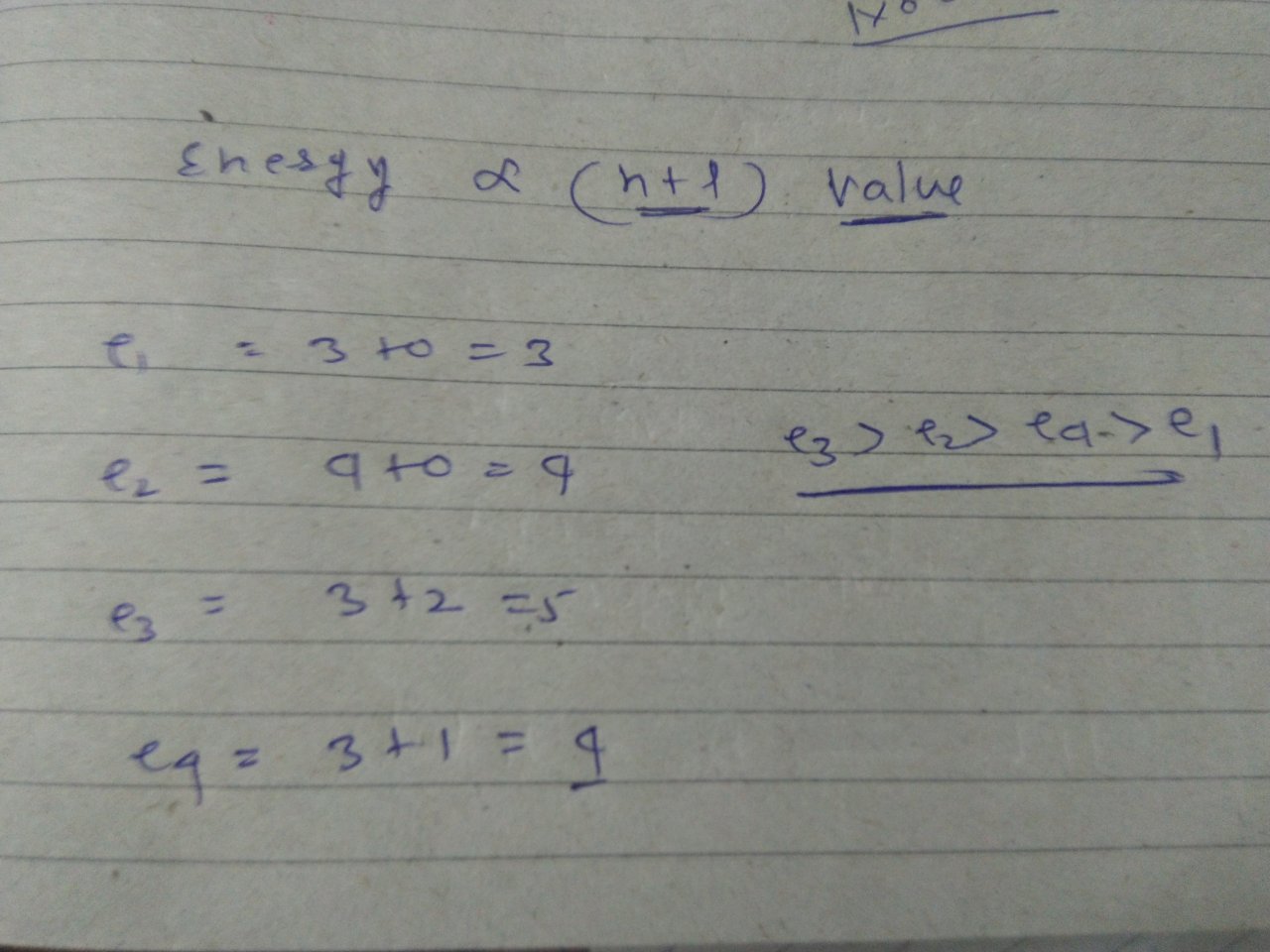
To speak on this question it is possible long.