The outer electronic configuration of gd
Doc 25 Pages. Sign in Open App. The outer electronic configuration of Gd Atomic number 64 is. Verified Answer.
Ionisation potential of hydrogen atom is Hydrogen atom is ground state is excited by monochromatic light of energy The spectral lines emitted by hydrogen according to bohr's theory will be-. Which one of the following is associated with a de Broglie wave of longer wavelength-a proton or an electron moving with same velocity? Maximum deviation from ideal gas is expected in case of-. The outer electronic configuration of Gd At. The outer electronic configuration of Gd Atomic no.
The outer electronic configuration of gd
This action cannot be undone. This will permanently delete All Practiced Questions. In the long form of periodic table, the elements having lowest ionization potential are placed in:. Elements with an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 3 belong to the group :. Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states? Are you sure? Clear Question Attempted. Botany All. Chemistry All. Physics All.
Subtopic: Atomic Size. A stream of electrons from a heated filament was passed between two ch The correct set of four quantum numbers of the valence electrons of ru
The lanthanoid follow the 4f 5d 6s 2 configuration common configuration with some exception due to full filled half filled electronic configuration. JEE Main session 2 registration ends tomorrow; options to login, image instructions. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M.
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now. We receieved your request Stay Tuned as we are going to contact you within 1 Hour. Thank you for registering.
The outer electronic configuration of gd
Gadolinium is a classified lanthanide element. In this article, I have discussed in detail how to easily write the complete electron configuration of gadolinium. The total number of electrons in gadolinium is sixty-four. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in gadolinium in specific rules in different orbits and orbitals is called the electron configuration of gadolinium. The electron configuration of gadolinium is [ Xe ] 4f 7 5d 1 6s 2 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbitals follows different principles.
Milla jasmine world star
Latest Question A sum of money under compound interest doubles itself in 4 years. Learn Change. Which of the following electronic configuration is not possible-. Have you? Doc 25 Pages. Continue with Facebook. Copper 2. Are you sure? H, O, N. Online Courses and Certifications Change. The energies E 1 and E 2 of two radiations are 25eV and 50eV respect What is the maximum number of electrons that can be associated with th What is the maximum number of orbitals that can be identified with the
The commonly used long form of the periodic table is designed to emphasize electron configurations. Since it is the outermost valence electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building-up process is of far more interest to a chemist than the first.
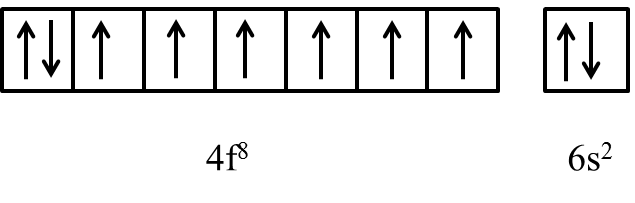
View All Videos. Already Have an Account? The Question and answers have been prepared according to the JEE exam syllabus. Physics All. Medicine and Allied Sciences Change. Na, K, Rb 4. Continue with Google. Question Type. Zn and Cd do not show variable valency like 'd' block elements due to : 1. View courses related to this question. Besides giving the explanation of The outer electronic configuration of Gd Atomic number 64 is AIEEE a 4f3, 5d5, 6s2b 4f8,d0, 6s2c 4f4,5d4,6s2d 4f7, 5d1,6s2Correct answer is option 'D'. Elements with an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 3 belong to the group : 1. Was this answer helpful? Germanium 3.

I congratulate, a remarkable idea