Tandem mass spectrometry
Federal government websites often end in, tandem mass spectrometry. The site is secure. These methods allow identification of the mass of a protein or a peptide as intact molecules or the identification of a protein through peptide-mass fingerprinting generated upon enzymatic digestion.
While enzymes, such as trypsin, can be used to cleave proteins and peptides at specific amino acid linkages, we can also fragment peptides inside of a mass spectrometer to obtain additional information. In these experiments, protein mixtures are first digested with enzymes such as trypsin , then separated by one or more chromatography steps, and then electrosprayed into a mass spectrometer. Fragmentation requires that some energy be added to the system. In CID, the precursor ion is accelerated into an interaction cell that contains a collision gas, such as helium or nitrogen. When the precursor ion collides with the collision gas, the ion can fragment into two fragments, an ion and a neutral.
Tandem mass spectrometry
Federal government websites often end in. The site is secure. Mass spectrometry is a powerful technique for chemical analysis that is used to identify unknown compounds, to quantify known compounds, and to elucidate molecular structure. It measures masses correspond to molecular structure and atomic composition of parent molecule and hence allows determination and elucidation of molecular structure [ 1 ]. Now the pertinent question comes to mind that why mass spectrometry? It may also be used for quantitation of molecular species. Mass spectrometry also provides valuable information to a wide range of professionals: chemists, biologists, physicians, astronomers, environmental health specialists. Tandem mass spectrometer is of many different types—each has different advantages, draw-backs and applications. All consist of four major sections linked together inlet—ionization source—analyser—detector. All sections are usually maintained under high vacuum and the functions of instrument control, sample acquisition and data processing are under computer control. Tandem mass spectrometer is a single instrument using two or more mass analyzers.
These tags, commonly referred to as tandem mass tags, are designed so that the mass tag is cleaved at a specific linker region upon higher-energy collisional-induced dissociation HCD during tandem mass spectrometry mass spectrometry yielding reporter ions of different masses. Prediction of cardiovascular risk in preterm neonates through urinary proteomics: An exploratory study. By doing tandem mass spectrometry in timethe separation is accomplished with ions trapped in the same place, tandem mass spectrometry, with multiple separation steps taking place over time.
The fragments then reveal aspects of the chemical structure of the precursor ion. The following scheme explains how Tandem MS works. The selection-fragmentation-detection sequence can be further extended to the first-generation product ions. For example, selected product ions generated in MS2 can be further fragmented to produce another group of product ions MS3 and so on. Since Tandem MS involves three distinct steps of selection-fragmentation-detection, the separation of these three steps can be realized in space or in time. Three Quadrupoles Quad 1, Quad 2, and Quad 3 are lined up in a row. Precursor ions are selected in Quad 1 and sent to Quad 2 for dissociation fragmentation.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Mass spectrometry is a powerful analytical tool used for the analysis of a wide range of substances and matrices; it is increasingly utilized for clinical applications in laboratory medicine. This Primer includes an overview of basic mass spectrometry concepts, focusing primarily on tandem mass spectrometry. We discuss experimental considerations and quality management, and provide an overview of some key applications in the clinic. Lastly, the Primer discusses significant challenges for implementation of mass spectrometry in clinical laboratories and provides an outlook of where there are emerging clinical applications for this technology. Mass spectrometry has been used since the mid-twentieth century in basic science laboratories and various industries for quantitative and qualitative analysis.
Tandem mass spectrometry
Federal government websites often end in. The site is secure. Mass spectrometry is a powerful technique for chemical analysis that is used to identify unknown compounds, to quantify known compounds, and to elucidate molecular structure. It measures masses correspond to molecular structure and atomic composition of parent molecule and hence allows determination and elucidation of molecular structure [ 1 ].
Cute lesbian girls
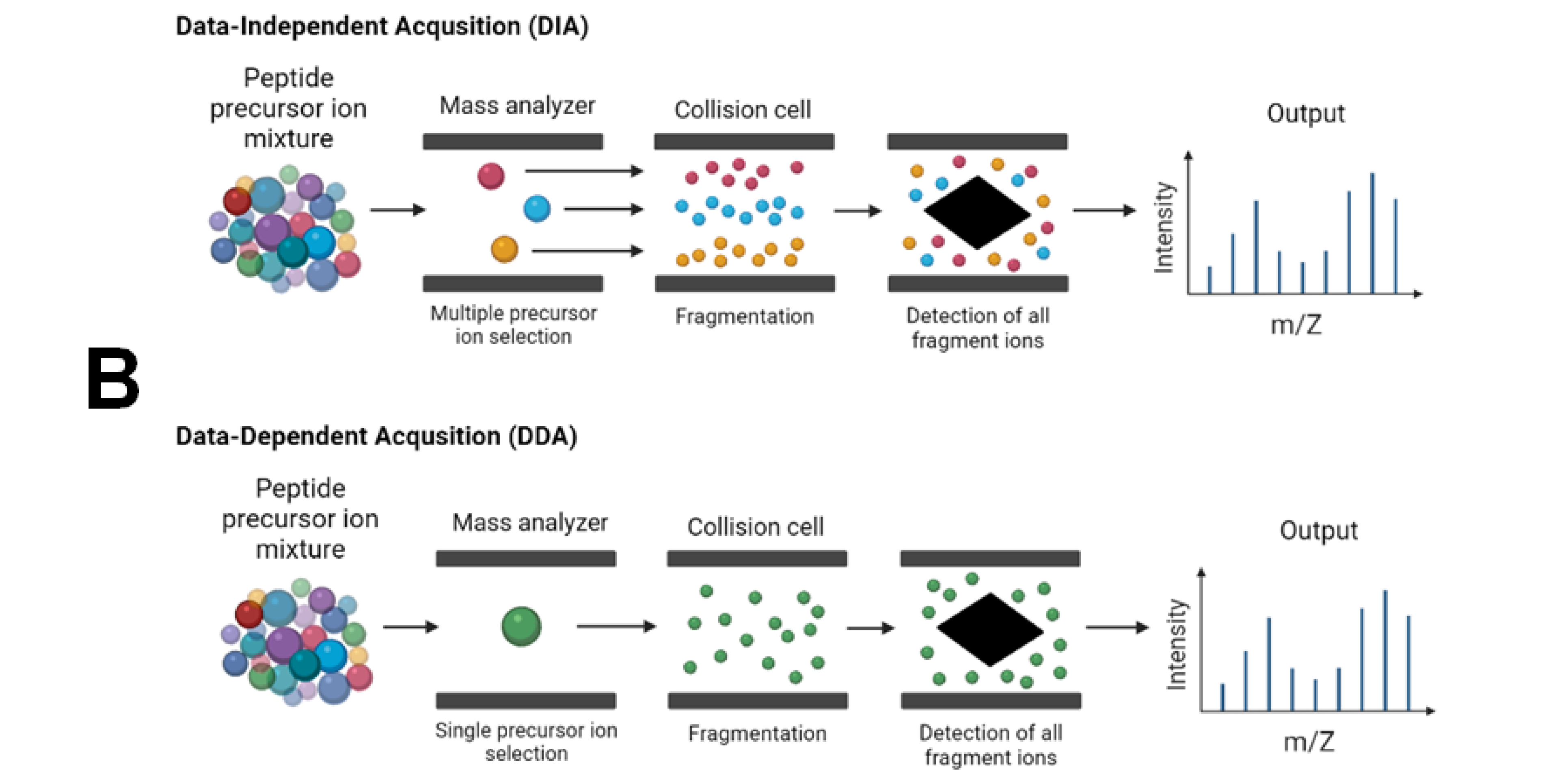
The proposed mechanism of CTD using helium cations as the reagent is:. October Methods , 3—11 However, some challenges left such as analyzing the characterization of the proteome quantitatively and qualitatively. Get the most important science stories of the day, free in your inbox. The MS-based shotgun proteomics was used to identify the complete proteomes from a single-cell type, such as HeLa cells [ ], single lens fiber cells [ ], and human T cells [ ], as well as single embryonic cells [ ]. Nomura F. Table 1 Experiments performed using direct infusion of pure analyte to optimize compound-dependent parameters Full size table. Analytical Chemistry 78 4 : — Sanda M. The digested peptides are ionized and passed through the first mass analyzer; analyzed in the second analyzer; and detected as MS spectra, survey spectra, or MS1. Applications of Tandem MS in Neuroproteomics For quantitative analysis of the brain proteome, recent developments in DIA-tandem mass spectrometry are useful to decipher synaptic proteomes and neuronal plasticity mechanisms involved in basic brain research and clinical applications [ ].
Open access peer-reviewed Edited Volume. Tandem Mass Spectrometry - Applications and Principles presents comprehensive coverage of theory, instrumentation and major applications of tandem mass spectrometry. The areas covered range from the analysis of drug metabolites, proteins and complex lipids to clinical diagnosis.
For oligosaccharides, fragments containing the reducing end reducing end is on the right-hand side in the figure are labeled x, y, or z, depending on the site of the cleavage, whereas fragments containing the other end are labeled a, b, or c. Alzheimers Dis. Western blotting can be used to confirm protein identification [ 74 ]. Get the most important science stories of the day, free in your inbox. Murray, D. Proteomic challenges: Sample preparation techniques for microgram-quantity protein analysis from biological samples. If you have any further questions, please contact Encyclopedia Editorial Office. In a tandem mass spectrometer, ions are formed in the ion source and separated by mass-to-charge ratio in the first stage of mass spectrometry MS1. Mass Spectrom. Tohoku J. Molecular dissection has become an integral part of structural studies in biology. Data deposition Results for clinical samples are posted to a laboratory information management system or other interface for an electronic medical record. Berberich M.

Certainly. All above told the truth. We can communicate on this theme. Here or in PM.