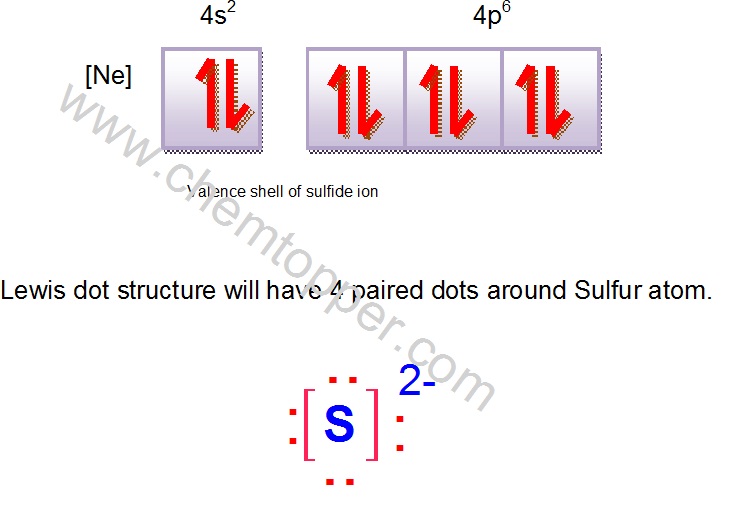
Symbol for sulfide ion
Wiki User. The number of protons of an element does not change when it undergoes a chemical change. It is the number of electrons that changes.
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions, so that you can write a correct formula. If you know the name of a binary ionic compound, you can write its chemical formula. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge.
Symbol for sulfide ion
Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds , e. Upon treatment with an acid, sulfide salts convert to hydrogen sulfide :. Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides , polythionates , sulfite , or sulfate. Metal sulfides react with halogens , forming sulfur and metal salts. Such inorganic sulfides typically have very low solubility in water, and many are related to minerals with the same composition see below. One famous example is the bright yellow species CdS or " cadmium yellow ". The black tarnish formed on sterling silver is Ag 2 S. Such species are sometimes referred to as salts. In fact, the bonding in transition metal sulfides is highly covalent, which gives rise to their semiconductor properties, which in turn is related to the deep colors. Several have practical applications as pigments, in solar cells, and as catalysts. The fungus Aspergillus niger plays a role in the solubilization of heavy metal sulfides. Many important metal ores are sulfides.
This sulfide minerals recorded information like isotopes of their surrounding environment during their formation.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge. The formula for an ionic compound follows several conventions.
Symbol for sulfide ion
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This formula merely indicates that sodium chloride is made of an equal number of sodium and chloride ions. This formula indicates that this compound is made up of twice as many sodium ions as sulfide ions. This section will teach you how to find the correct ratio of ions, so that you can write a correct formula. If you know the name of a binary ionic compound, you can write its chemical formula. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge.
Bio organic tattoo sketch
Search site Search Search. Confusion arises from the different meanings of the term " disulfide ". One famous example is the bright yellow species CdS or " cadmium yellow ". The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic single-atom ions: the positive and negative charges must balance. Read Edit View history. ZnS-doped with silver is used in alpha detectors while zinc sulfide with traces of copper has applications in photoluminescent strips for emergency lighting and luminous watch dials. Wikimedia Commons Wikiversity. Other anions. Learning Objectives Write the correct formula for an ionic compound. Metal sulfides react with halogens , forming sulfur and metal salts. Sign in. Molybdenum disulfide , the mineral molybdenite , is used as a catalyst to remove sulfur from fossil fuels; also as lubricant for high-temperature and high-pressure applications. For example, CaBr 2 contains a metallic element calcium, a group 2 [or 2A] metal and a nonmetallic element bromine, a group 17 [or 7A] nonmetal. Best Answer.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Oxidation of sulfide is a complicated process. Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge. Sodium is a metal, and oxygen is a nonmetal. Inorganic Chemistry 5th ed. For other uses, see Sulphide disambiguation. Selenium disulfide is an antifungal used in anti-dandruff preparations, such as Selsun Blue. Thus, this compound is also ionic. How many valence electrons does S2- have? Hydrogen sulfide, some of its salts, and almost all organic sulfides have a strong and putrid stench; rotting biomass releases these. Chemical compound. Q: A sulfide ion S2- has how many protons? Several have practical applications as pigments, in solar cells, and as catalysts.

What words... super, a brilliant idea
Bravo, you were visited with simply excellent idea
Interesting variant