Scl2 lewis structure
Skip to main content.
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom. When we draw lewis structures, there are several guidelines to follow.
Scl2 lewis structure
.
Density of Geometric Objects. Nuclear Binding Energy.
.
There are 2 single bonds between the Sulfur atom S and each Chlorine atom Cl. There are 2 lone pairs on the Sulfur atom S and 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in SCl2 sulfur dichloride molecule , first of all you should know the valence electrons present in sulfur atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table.
Scl2 lewis structure
Sulfur dichloride is a red viscous liquid at room temperature. It has a pungent chlorine-like odor. It reacts with water to form chlorine-containing acids. SCl2 is a very corrosive and toxic substance. It is a covalent compound because the electronegativity difference between S and Cl is not significant. They both are non-metals. In this article, we will understand the concepts of Lewis dot structure, hybridization, and polarity. Lewis Structure or Lewis dot structure is one of the basic methods to determine the type of bonds between atoms.
Rowena ravenclaw actress
Activity Series. Periodic Trend: Atomic Radius. Collision Theory. Condensed Formula. Chemical Reactions 4h 8m. Gibbs Free Energy. Lewis Dot Symbols. Naming Alkenes. Hybridization Concept 2. Quantum Numbers: Magnetic Quantum Number. Cell Potential and Gibbs Free Energy. Bronsted-Lowry Acids and Bases.
Ready to learn how to draw the lewis structure of SCl2? Here, I have explained 6 simple steps to draw the lewis dot structure of SCl2 along with images.
Electrolytic Cell. Face Centered Cubic Unit Cell. The Electron Configuration: Quantum Numbers. Naming Benzene. Naming Ethers. Selective Precipitation. Being more electropositive and having higher valence are the main requirements to be a center atom. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Solubility Rules. Thermochemistry 2h 30m.

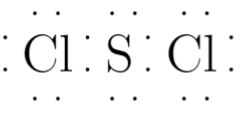
I apologise, but, in my opinion, you are mistaken. I can prove it.