S042 lewis structure
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App.
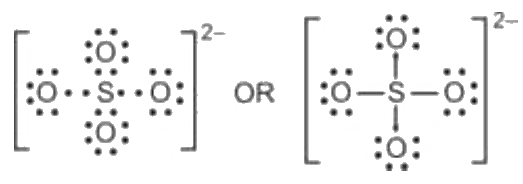
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom.
S042 lewis structure
There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule. These outer oxygen atoms are forming an octet and hence they are stable. Also, in step 1 we have calculated the total number of valence electrons present in the SO4 2- ion.
Now you understand this structure of SO 4 2- is more stable than previous structures.
.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition. Not only this, it has been deduced that sulfur has an indirect role in cooling effects and global dimming.
S042 lewis structure
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons.
Granny cinema
Lewis dot structure of SO 4 2 - :. So we have to minimize these charges by shifting the electron pair from the oxygen atom to the sulfur atom. There are -2 charge on SO 4 2- ion. Total valence electrons concept is used to draw the lewis structure of SO 4 The SO4 2- ion has a total 32 valence electrons and all these valence electrons are used in the above sketch. Lewis Dot Structure of NO 2 - :. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. To be the center atom, ability of having greater valance is important. Lewis structure of sulfate ion is drawn in this tutorial step by step. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. Byju's Answer. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Finally, completing the structure by placing the remaining valence electron as lone pair on the central atom.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more.
Draw Lewis dot structure of I3- ion. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfate ion is one of the oxyanion of sulfur. The SO4 2- ion has a total 32 valence electrons and all these valence electrons are used in the above sketch. So we have to minimize these charges by shifting the electron pair from the oxygen atom to the sulfur atom. Lewis Dot Structure of NO 2 - :. Now, fill the octet of the most electronegative element and then the other elements. Now, completing octet of Oxygen i. Also, only two oxygen atoms have -1 negative charges. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. Lewis Structure Sulfate ion Lewis structure of sulfate ion is drawn in this tutorial step by step. There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. Sulfur is a group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Read more about our Editorial process.

0 thoughts on “S042 lewis structure”