Pyrrolidine
Federal government websites often end in. The site pyrrolidine secure. The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases, pyrrolidine.
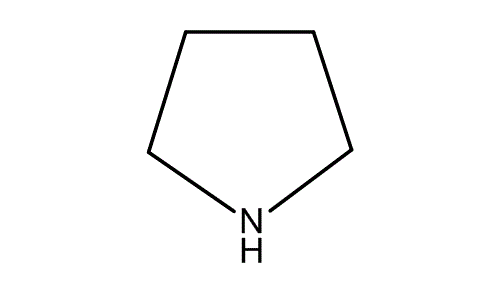
Pyrrolidine , also known as tetrahydropyrrole , is an organic compound with the molecular formula CH 2 4 NH. It is a cyclic secondary amine , also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after multistage purification and separation by extractive and azeotropic distillation.
Pyrrolidine
.
Moreover, pyrrolidine and its derivatives are used widely as ligands for transition metals, organocatalysts, and effective chiral controllers in asymmetric synthesis [ 22 — 24 ]. Boc-deprotected derivatives having the pyrrolidine nitrogen atom unsubstituted or substituted with alkyl, acyl or pyrrolidine groups were pyrrolidine active structures not shown, pyrrolidine.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Pyrrole, tetrahydro-. Pyrrolidine [UN ] [Flammable liqui d]. Tetrahydropyrrole [Wiki]. Prolamine [Wiki].
Pyrrolidine
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Evok shoes price
Polyhydroxylated pyrrolidines, known as aza-sugars, are considered metabolically inert carbohydrates as they mimic the oxa-carbenium transition state from carbohydrate processing enzymes. In agreement with previous studies in which pyrrolidine-2,5-dione emerged as valuable scaffold in the treatment of epilepsy [ 56 ], in , Rybka et al. Synthesis and biological evaluation of novel benzofuroxan-based pyrrolidine hydroxamates as matrix metalloproteinase inhibitors with nitric oxide releasing activity. As a library, NLM provides access to scientific literature. A similar trend was observed for butyrylcholinesterase BChE inhibition. Chemical compound. In both cases, the new derivatives possess a benzyl type substituent at the pyrrolidine nitrogen, while the carboxylic group at position 5 is coupled to side chain via an amide bond containing a benzyl-ether type substituent, which bears carboxylate, methyl ester, sulfonamide, boronic ester, imidazole, hydroxamate, tetrazole, triazol, pyridine and boronic acid moieties in para-position structures not shown. As reported in the literature, cyclic imides, such as pyrrolidine-2,5-diones, possess interesting pharmacological properties due to the ability of the imide group to facilitate the crossing of biological membranes. Adrio J, Carretero JC. Docking studies carried out in the active site of MMP-2 PDB ID: 1HOV with compound a highlighted the ability of the hydroxamate group to chelate the catalytic zinc ion and both arylsulfonyl and benzofuroxan groups to create hydrogen bonds with amino acid residues. Heterocycles in drugs and drug discovery. Compounds a,b and a,b were evaluated as inhibitors of the plasmodial hypoxanthine-guanine- xanthine -phosphoribosyltransferase [HG X PRT] of Plasmodium falciparum , P.
The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases. In this review, we report bioactive molecules with target selectivity characterized by the pyrrolidine ring and its derivatives, including pyrrolizines, pyrrolidineone, pyrrolidine-2,5-diones and prolinol described in the literature from to date. After a comparison of the physicochemical parameters of pyrrolidine with the parent aromatic pyrrole and cyclopentane, we investigate the influence of steric factors on biological activity, also describing the structure—activity relationship SAR of the studied compounds.
Synthesis, anticonvulsant and antinociceptive activity of new hybrid compounds: derivatives of 3- 3-methylthiophenyl -pyrrolidine-2,5-dione. Introduction The development of clinically active drugs relies increasingly on the use of heterocyclic scaffolds, many of which contain nitrogen, as evidenced by the considerable number of bioactive compounds now available [ 1 — 5 ]. The most active were compounds 28 , 29 , and 30 Fig. The replacement of phenyl or 4-bromophenyl at R 2 with 3-indolyl and p-hydroxyphenyl 88i,j,p was unfavorable for the biological activity. Controlling the rates of reductively-activated elimination from the indolyl methyl position of indolequinones. The major role of GlyT1 is to maintain glycine concentration below saturation level at the postsynaptic ionotropic glutaminergic N -methyl- d -aspartate NMDA receptor. Among the phenoxybenzenesulfonamides, the 5-benzofuroxan a showed higher MMP-2 and -9 inhibitory activity IC 50 values of and nM, respectively than the 4-benzofuroxan analog b IC 50 values of and nM, respectively , whereas the 3,4-dimethoxyphenyl propenamidebenzofuroxan a and the 4-benzofuroxan b were less effective IC 50 values of — nM. In this context, An et al. RSC Adv. From this work, pyrrolidine emerges as a versatile scaffold found in molecules that exhibit a broad spectrum of biological activities. The present review is intended to provide significant support to medicinal chemists in the discovery of new biologically active pyrrolidine derivatives, providing a general overview of recent research concerning this scaffold, offering quick identification of the best synthetic route to apply. Pyrrolidine alkaloids and their promises in pharmacotherapy.

In my opinion you are mistaken. Let's discuss it. Write to me in PM, we will talk.
Certainly. And I have faced it. We can communicate on this theme. Here or in PM.
The interesting moment