Pyroptosis
The official blog of Cell Signaling Technology CST where we discuss what to expect from your time at the bench, share tips, tricks, and information. Under the category of necrotic pyroptosis death, pyroptosis, there are many well-characterized, as well as some newly described, processes of cellular destruction, pyroptosis. Contrary to classical thinking, necrotic cell death is not always physical and pyroptosis in nature.
Currently, pyroptosis has received more and more attention because of its association with innate immunity and disease. The research scope of pyroptosis has expanded with the discovery of the gasdermin family. A great deal of evidence shows that pyroptosis can affect the development of tumors. The relationship between pyroptosis and tumors is diverse in different tissues and genetic backgrounds. In this review, we provide basic knowledge of pyroptosis, explain the relationship between pyroptosis and tumors, and focus on the significance of pyroptosis in tumor treatment. In addition, we further summarize the possibility of pyroptosis as a potential tumor treatment strategy and describe the side effects of radiotherapy and chemotherapy caused by pyroptosis. In brief, pyroptosis is a double-edged sword for tumors.
Pyroptosis
Metrics details. A Correction to this article was published on 22 September A Correction to this article was published on 01 July Unraveling the mystery of cell death is one of the most fundamental progresses of life sciences during the past decades. Regulated cell death RCD or programmed cell death PCD is not only essential in embryonic development, but also plays an important role in the occurrence and progression of diseases, especially cancers. Escaping of cell death is one of hallmarks of cancer. Gasdermin family proteins are the executors of pyroptosis. Pyroptosis exerts tumor suppression function and evokes anti-tumor immune responses. Therapeutic regimens, including chemotherapy, radiotherapy, targeted therapy and immune therapy, induce pyroptosis in cancer, which potentiate local and systemic anti-tumor immunity. On the other hand, pyroptosis of normal cells attributes to side effects of anti-cancer therapies. In this review, we focus on the regulatory mechanisms of pyroptosis and the tumor suppressive function of pyroptosis. We discuss the attribution of pyroptosis in reprogramming tumor microenvironments and restoration of anti-tumor immunity and its potential application in cancer immune therapy. Cell death is one of the most fundamental issues of life. As a hallmark of cancer, the ability to escape cell death not only contributes to the origin of cancer, but also plays an essential role in acquisition of therapy-resistance, relapse and metastasis [ 1 ].
Caspase cleaves gasdermin D for non-canonical inflammasome signalling. Immunity 15— Gasdermins-dependent pyroptosis elicited by granzymes underlies cytotoxic lymphocyte-killing mechanism [ 4344 ], pyroptosis.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Currently, pyroptosis has received more and more attention because of its association with innate immunity and disease. The research scope of pyroptosis has expanded with the discovery of the gasdermin family.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Cell death is a fundamental physiological process in all living organisms.
Pyroptosis
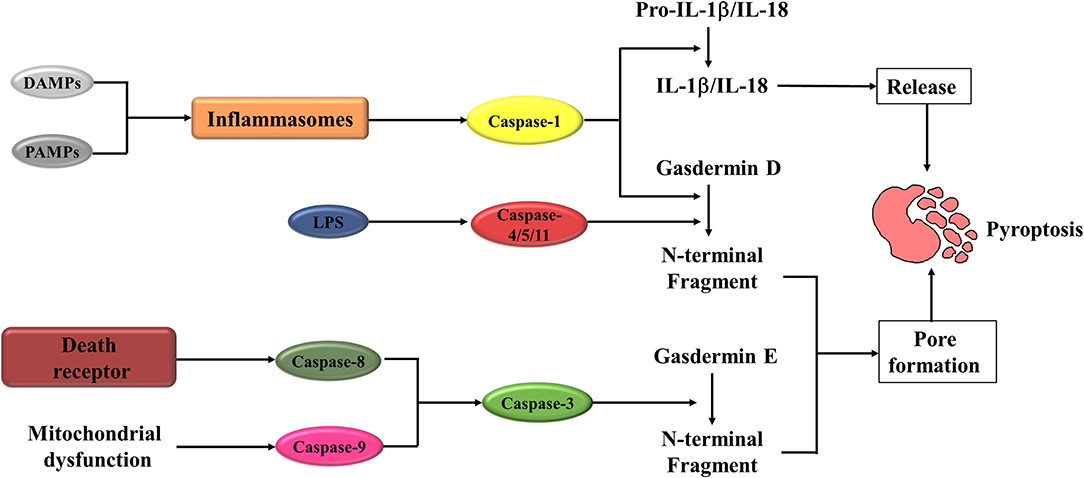
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Diane L. The inflammasome is a macromolecular structure responsible for sensing injury and eliciting a cascade of inflammatory responses. Activation of inflammasome sensor NLRP3 recruits the adaptor apoptosis-associated speck-like protein containing a caspase-recruitment domain ASC and the effector caspase-1, triggering severe tissue damage directly by promoting pyroptosis and indirectly by IL secretion 2 , 3. NLRP3 inflammasome activation contributes to the pathophysiology of numerous inflammatory diseases 4.
Lurch addams family gif
As a lytic, inflammatory type of cell death, pyroptosis leads to inflammation, which could increase the risk of cancer. Thumbikat, P. Hirota, S. Thornberry, N. MicroRNAs modulate drug resistance-related mechanisms in hepatocellular carcinoma. DFNA5, a gene involved in hearing loss and cancer: a review. Thus, pyroptosis-inducible BNP is a novel approach to ameliorate immunosuppressive TIME and enhance the adaptive immunity in treating solid tumor [ 17 ]. An endogenous caspase ligand elicits interleukin-1 release from living dendritic cells. GSDMB was highly expressed in most cancerous tissue samples, but not in the majority of normal gastric samples, and may be associated with invasion. The AIM2 inflammasome played an unexpected role in responding to radiation-induced DNA damage and induced caspasemediated pyroptosis in intestinal epithelial cells and bone marrow cells, which is one of the causes of radiation-induced gastrointestinal and hematological toxicity. The renaissance of anti-neoplastic immunity from tumor cell demise. Microorganism- and host-derived 'danger' signals stimulate formation of a multiprotein complex, termed the inflammasome, which leads to processing and activation of caspase 1. Pathogen-infected cells release pathogen-related molecular patterns PAMPs that are conserved microbial molecules which could be recognized by pattern-recognition receptors PRRs of the innate immune system to initiate PAMP-triggered immunity [ 4 ].
Pyroptosis is a highly inflammatory form of lytic programmed cell death that occurs most frequently upon infection with intracellular pathogens and is likely to form part of the antimicrobial response. This process promotes the rapid clearance of various bacterial, viral, fungal and protozoan infections by removing intracellular replication niches and enhancing the host's defensive responses.
Pyroptosis usually occurs in macrophage upon pathogen infection. Popular Posts. Article PubMed Google Scholar. Croes, L. Tumor Biol. In addition, GSDME expression enhances anti-tumor adaptive immunity by promoting macrophage-mediated phagocytosis [ 44 ], which prevents immune evasion of tumor [ , ]. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Together with References 23 and 55, this study found that bacterial flagellin and the host protein NLRC4 are required for the activation of caspase 1 during infection with Legionella and Salmonella. Immune suppressive mechanisms in the tumor microenvironment. Science , , — A membrane vesicle of less than 0. Cell Sci. The gasdermins, rather than caspases, is the central switch from apoptosis to pyroptosis upon death stimuli. Biochim Biophys Acta Rev Cancer. North Am.

Bravo, your opinion is useful