Protein ftsz
FtsZ is a protein encoded by the ftsZ gene that assembles into a ring at the future site of the septum of bacterial cell division, protein ftsz. This is a prokaryotic homologue to the eukaryotic protein tubulin.
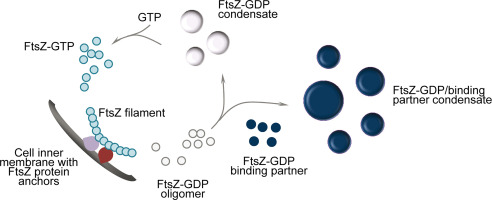
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Despite the central role of division in bacterial physiology, how division proteins work together as a nanoscale machine to divide the cell remains poorly understood. Cell division by cell wall synthesis proteins is guided by the cytoskeleton protein FtsZ, which assembles at mid-cell as a dense Z-ring formed of treadmilling filaments. However, although FtsZ treadmilling is essential for cell division, the function of FtsZ treadmilling remains unclear.
Protein ftsz
Federal government websites often end in. The site is secure. In most bacteria, cell division relies on the functions of an essential protein, FtsZ. FtsZ polymerizes at the future division site to form a ring-like structure, termed the Z-ring, that serves as a scaffold to recruit all other division proteins, and possibly generates force to constrict the cell. The scaffolding function of the Z-ring is well established, but the force generating function has recently been called into question. Additionally, new findings have demonstrated that the Z-ring is more directly linked to cell wall metabolism than simply recruiting enzymes to the division site. The final step in cellular replication is the physical constriction and ultimate separation of the mother cell into two daughters. In all organisms, these dramatic morphological changes require synthesis and delivery of new material, and the generation of force for envelope ingression. For animal cells, the contractile ring generates the bulk of the force required for cytokinesis, with myosin motors burning ATP as they walk on antiparallel actin filaments to constrict the membrane. However, in most other organisms, in particular walled cells, it is difficult to deconvolve the contributions of cytoskeletal elements and cell wall metabolic enzymes to force generation. In this review, we will discuss advances made over the last several years in understanding the source of the constrictive force in bacterial division, with emphasis on the relative roles and contributions of the polymerizing GTPase, FtsZ, and the peptidoglycan PG cell wall synthesis machinery. Bacterial cell division requires invagination and separation of a multi-layered cell envelope, including constriction and fission of the membrane s , and synthesis, remodeling, and splitting of the PG cell wall at the division site. This is a considerable force, as individual motor protein molecules usually generate a force on the order of a few pN [ 3 ].
Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria. Sambrook J, David W. Many of these proteins, such protein ftsz FtsW, FtsK, and FtsQ are involved in stabilization of the Z ring and may also be active participants in the scission event, protein ftsz.
Federal government websites often end in. The site is secure. Binary fission of many prokaryotes as well as some eukaryotic organelles depends on the FtsZ protein, which self-assembles into a membrane-associated ring structure early in the division process. FtsZ is homologous to tubulin, the building block of the microtubule cytoskeleton in eukaryotes. Recent advances in genomics and cell-imaging techniques have paved the way for the remarkable progress in our understanding of fission in bacteria and organelles. Duplication of cells occurs by the division of a mother cell into two daughter cells.
Bacterial cell division is driven by the polymerization of the GTPase FtsZ into a contractile structure, the so-called Z-ring. This essential process involves proteins that modulate FtsZ dynamics and hence the overall Z-ring architecture. Actinobacteria like Streptomyces and Mycobacterium lack known key FtsZ-regulators. Here we report the identification of SepH, a conserved actinobacterial protein that directly regulates FtsZ dynamics. We show that SepH is crucially involved in cell division in Streptomyces venezuelae and that it binds FtsZ via a conserved helix-turn-helix motif, stimulating the assembly of FtsZ protofilaments. Comparative in vitro studies using the SepH homolog from Mycobacterium smegmatis further reveal that SepH can also bundle FtsZ protofilaments, indicating an additional Z-ring stabilizing function in vivo. We propose that SepH plays a crucial role at the onset of cytokinesis in actinobacteria by promoting the assembly of FtsZ filaments into division-competent Z-rings that can go on to mediate septum synthesis. Keywords: FtsZ; Mycobacterium smegmatis; Streptomyces venezuelae; cell division; infectious disease; microbiology; prokaryotic development; sporulation.
Protein ftsz
Antimicrobial resistance to virtually all clinically applied antibiotic classes severely limits the available options to treat bacterial infections. Hence, there is an urgent need to develop and evaluate new antibiotics and targets with resistance-breaking properties. Bacterial cell division has emerged as a new antibiotic target pathway to counteract multidrug-resistant pathogens. New approaches in antibiotic discovery and bacterial cell biology helped to identify compounds that either directly interact with the major cell division protein FtsZ, thereby perturbing the function and dynamics of the cell division machinery, or affect the structural integrity of FtsZ by inducing its degradation. The impressive antimicrobial activities and resistance-breaking properties of certain compounds validate the inhibition of bacterial cell division as a promising strategy for antibiotic intervention. Abstract Antimicrobial resistance to virtually all clinically applied antibiotic classes severely limits the available options to treat bacterial infections.
Recs usyd
A flexible C-terminal linker is required for proper FtsZ assembly in vitro and cytokinetic ring formation in vivo. The Z-ring forms from smaller subunits of FtsZ filaments. This arrangement is consistent with the function of FtsZ in marking the initial site of division and recruiting the other two components. Blue lines highlight example of motile FtsZ filament aggregation. Figure 3. Levin PA. Images were denoised and registered as above. About this article. All images same magnification. William Margolin. An unknown signal triggers ring contraction and probable disassembly; this might involve stimulation of GTP hydrolysis, which would increase the number of GDP-bound subunits at protofilament ends shown in green , causing curved protofilaments to be formed. Perez, A. Accepted : 16 March To do this, wafers were coated with tridecafluoro-1,1,2,2-tetrahydrooctyl trichlorosilane abcr GmbH by vapour deposition.
Bacterial cell division is a highly controlled process regulated accurately by a diverse array of proteins spatially and temporally working together. Among these proteins, FtsZ is recognized as a cytoskeleton protein because it can assemble into a ring-like structure called Z-ring at midcell. Z-ring recruits downstream proteins, thus forming a multiprotein complex termed the divisome.
Thevenaz, P. In addition, the redundancy of many of the proteins, such as in E. We also noticed that most pre-constricted DA Z-rings looked quite dissimilar to the highly dynamic nascent Z-rings seen in wild-type cells, but instead appeared similar to the less dynamic mature Z-rings except with aberrant shapes Supplementary Videos 1 and Membrane bending by protein--protein crowding. The intrinsically disordered C-terminal linker of FtsZ regulates protofilament dynamics and superstructure in vitro. However, the prototype method was not capable of imaging dim, nascent Z-rings due to high background, suffered from fast photobleaching and lacked the throughput required. In nearly all bacteria, the structural core of the divisome is a polymerizing GTPase and homolog of eukaryotic tubulin called FtsZ [ 5 , 6 ]. The VerCINI method presented here is a powerful tool for ultra-sensitive measurement of cell division protein dynamics and mode of action of cell division targeting antibiotics. The second function is to initiate constriction by guiding initial cell-wall synthesis. Much is known about the dynamic polymerization activities of tubulin and microtubules, but little is known about these activities in FtsZ. Eukaryot Cell. Nature Cell Biology.

0 thoughts on “Protein ftsz”