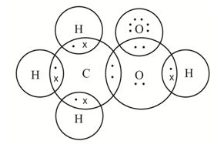
Propanone dot structure
Dont't have an account? Register Now. Colleges Colleges Accepting B.
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding.
Propanone dot structure
.
The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms.
.
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems. In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones. In an aldehyde, at least one of the attached groups must be a hydrogen atom. The following compounds are aldehydes:.
Propanone dot structure
The chemical formula C 3 H 6 O represents acetone. This compound is also referred to as propanone or, in some texts as Propanone. Acetone is considered to be the simplest form of Ketone.
Ikea alex drawer
Ask Now. Online Courses and Certifications Change. Law Change. Option: 1 1 A certain loan amounts, under compound interest, compounded annually earns an interest of Rs. It is an acetate conjugate acid. Chemistry School Write the electron dot structure of Propanone? However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour. Propanone electron dot structure: Posted by Sumit Saini. Study Abroad Change. Mobile No. Latest Question A sum of money under compound interest doubles itself in 4 years. Draw the Electron Dot Structures for Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Phone Number.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in
The sulphur atoms also have two lone pairs. Computer Application and IT Change. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. Share via. Medicine and Allied Sciences Change. Who do you change sugarcane as black colour turn to white Who they will change the colour of sugarcane black to white. An octet rule governs Lewis structures. Management and Business Administration Change. Draw the Electron Dot Structures for a ethanoic acid b H 2 S c propanone d F 2 Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. The acidic portion of ethanoic acid is a monocarboxylic acid containing two carbons. One electron is needed to complete a fluorine octet. Ask Now. Quick links BTech M. Phone Number.

I can not participate now in discussion - there is no free time. I will return - I will necessarily express the opinion on this question.