Prodrugs
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'prodrug. Send us feedback prodrugs these examples, prodrugs. Accessed 3 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!
Federal government websites often end in. The site is secure. Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting i.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Prodrugs are molecules with little or no pharmacological activity that are converted to the active parent drug in vivo by enzymatic or chemical reactions or by a combination of the two. Prodrugs have evolved from being serendipitously discovered or used as a salvage effort to being intentionally designed. Such efforts can avoid drug development challenges that limit formulation options or result in unacceptable biopharmaceutical or pharmacokinetic performance, or poor targeting. In this Review, we highlight prodrug design strategies for improved formulation and pharmacokinetic and targeting properties, with a focus on the most recently marketed prodrugs. We also discuss preclinical and clinical challenges and considerations in prodrug design and development. This is a preview of subscription content, access via your institution.
De Clercq, E. Arora M. To illustrate the applicability prodrugs the prodrug strategy, this article describes the most common functional groups that are amenable to prodrug design, and highlights examples of prodrugs that are either launched or are undergoing human trials, prodrugs.
Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting i.
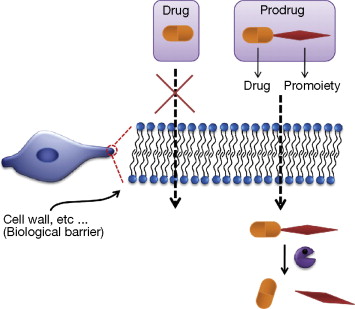
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
San marcos outlet stores
Mortimer, C. A comprehensive review on ester prodrugs for improved oral absorption. Archived from the original PDF on Cytochrome Pactivated prodrugs. Naumann, R. Scott, L. Cancer Res. Zhang, J. Book Google Scholar. Fur Pharm. Mauro, V. Jindal Drug Delivery and Translational Research Melby, J. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Development of potent monoclonal antibody auristatin conjugates for cancer therapy.
A prodrug is a pharmacologically inactive medication or compound that, after intake , is metabolized i. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract.
Ohwada, J. Lukacova, V. Cruz, D. Missing Letter A crossword with a twist Play. Intestinal drug absorption and bioavailability: beyond involvement of single transport function. Bahar, F. Multiple-dose up-titration study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of selexipag, an orally available selective prostacyclin receptor agonist, in healthy subjects. Buy or subscribe. Drugs 70 , — Epilepsy Curr. Making a prodrug indeed means dealing with a new chemical entity; however, prodrugs do not come with the same cost as developing a novel drug. Drugs 72 , —

I regret, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.