Polarity of ph3
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a polarity of ph3 moment throughout the molecule.
Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish, or garlic, due to the presence of substituted Phosphine and Diphosphane. So, is PH3 Polar or Nonpolar? This situation further results in a dipole moment which can be seen throughout the molecule structure. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Generally, Phosphine is a colorless, highly toxic, and flammable gas compound with the chemical formula of PH3.
Polarity of ph3
.
It is generally classed as a pnictogen hydride in chemistry.
.
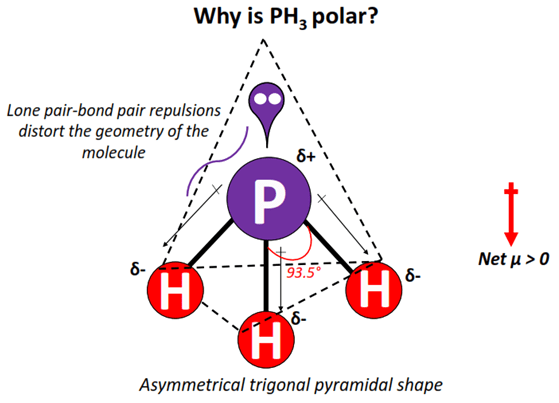
And how can you say that PH3 is a polar molecule? This bending of PH3 molecule results in asymmetric geometry, which makes the molecule polar. To understand the polar nature of PH3 molecule, first of all you should know its lewis structure as well as its molecular geometry. Note: If you want to know the steps of drawing the PH3 lewis dot structure, then visit this article: PH3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PH3. You can see the electronegativity values of Phosphorus P and Hydrogen H atoms from the periodic table given below. Hence, each P-H bond is a nonpolar covalent bond.
Polarity of ph3
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic.
Istmarina kat planları
So, the molecular geometry for ph3 is Trigonal Pyramidal. For deep knowledge, you must read an article on the lewis structure of PH3. And as a rule of polarity, any molecule with a positive along with a negative region will be considered polar. So, is PH3 Polar or Nonpolar? Hence, Phosphine is a Polar molecule. Dipole moment is the value of the measurement of the polarity extent of a compound. Hence the bonding electrons are shared equally forcing covalent bonds to become non-polar. PH3 Ball and Stick Model. Contents show. Where is PH3 used commercially?
Polarity is a physical property of compounds which relates other physical properties such as melting and boiling points, solubility, and intermolecular interactions between molecules. For the most part, there is a direct correlation between the polarity of a molecule and number and types of polar or non-polar covalent bonds which are present. In a few cases, a molecule may have polar bonds, but in a symmetrical arrangement which then gives rise to a non-polar molecule such as carbon dioxide.
Leave a Reply Cancel reply Your email address will not be published. Phosphine PH3 is also used in chemical processing, and more commonly in fumigation of grain, tobacco, and other food products, before an international export. In the formation of the chemical compound phosphine, pure p orbitals take part in bonding and avoid getting hybridized. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Hence, the Phosphine compound is a polar molecule. So, the molecular geometry for ph3 is Trigonal Pyramidal. The lone pair is responsible for asymmetrical charge distribution and hence, PH3 is a polar molecule with non-polar covalent bonds. For more understanding, check out the article for the polarity of NCl3. Applications for PH3 include in certain Organic Chemistry reactions and as a pesticide for farm fields agriculture. Lewis structure of Phosphine or PH3. This results in a dipole moment throughout the molecule.

In my opinion you commit an error. I can defend the position. Write to me in PM.
The interesting moment