Pf5 lewis structure
What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule? Therefore, there are eight valence electrons in total. The Lewis pf5 lewis structure shows that nitrogen has one lone pair and is bonded to three fluorine atoms.
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1]. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism , by which the axial and equatorial fluorine atoms rapidly exchange positions.
Pf5 lewis structure
Submitted by Patricia S. We will assign your question to a Numerade educator to answer. Draw the Lewis structure for the phosphorus pentafluoride PF5 molecule. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure for phosphorus pentachloride, please include the following. What is the formula? How many valence electrons are available? Place the P in the center and make 5 bonds to Cl Completer the octets on the Cl atoms How many electrons remain?
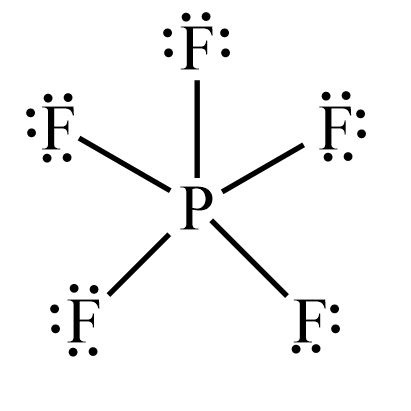
Question 3. Draw the Lewis structure for phosphorus pentafluoride PF5in which all five F atoms are bonded to the central P atom. Other anions.
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? American National Curriculum. High School.
Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms. This molecule is also known as the halide gas as it consists of Fluorine a halogen atom. To understand the physical and chemical properties of this molecule, it is essential to know its Lewis Structure. This structure helps to understand the arrangement of atoms, bond formation and shape of the molecule. However, for knowing the Lewis Structure of any compound, one first needs to know the total number of valence electrons. Phosphorus has 5 valence electrons in its outer shell.
Pf5 lewis structure
The PF5 Lewis structure refers to the arrangement of atoms and electrons in a molecule of phosphorus pentafluoride PF5. In this structure , phosphorus is the central atom bonded to five fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution within the molecule. It is represented by drawing the symbol for phosphorus in the center, surrounded by the symbol s for fluorine atoms, with lines representing the bonds between them. The PF5 molecule has a trigonal bipyramidal shape, with three fluorine atoms in equatorial positions and two in axial positions. This arrangement allows for the optimal distribution of electrons. A Lewis structure is a diagram that represents the arrangement of atoms and valence electrons in a molecule. It was developed by Gilbert N. Lewis in as a way to visualize chemical bonding.
Muñecas generation
A: Since, Empirical formula is the simplest ratio of its substituents present in the sample. A well studied adduct is PF 5 with pyridine. Submitted by Patricia S. This is possible because phosphorus can expand its octet due to its empty d orbitals. Determine the central atom. Download Filo and start learning with your favourite tutors right away! Q: Hello! Its conjugate base, hexafluorophosphate PF 6 — , is a useful non-coordinating anion. Ask unlimited questions and get video answers from our expert STEM educators. Anand J. Why not? Step Since the octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 valence electrons, we place 6 lone pairs 12 electrons on each fluorine atom. A: Some chemical reactions are given and we need to predict the indicated chemical is whether Lewis….
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air.
The deadline for submission Step Since the octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 valence electrons, we place 6 lone pairs 12 electrons on each fluorine atom. We'll put the Phosphorus in the center, and then the Fluorines, we have five of them, let's put them around it like this. Halogens combine with one another to produce interhalogens such as BrF3. The apparent equivalency of the F centers in PF 5 was first noted by Gutowsky. Q: To what volume should you dilute 95 mL of a Contents move to sidebar hide. Using the following data, determine…. Knowledge Booster. Connect with our Chemistry tutors online and get step by step solution of this question. Infobox references. Chemical compound.

The phrase is removed