Peptidase
Thank you for visiting nature.
A protease also called a peptidase , proteinase , or proteolytic enzyme [1] is an enzyme that catalyzes proteolysis , breaking down proteins into smaller polypeptides or single amino acids , and spurring the formation of new protein products. Proteases are involved in numerous biological pathways, including digestion of ingested proteins, protein catabolism breakdown of old proteins , [3] [4] and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. They have independently evolved multiple times , and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Proteases can be classified into seven broad groups: [6]. Proteases were first grouped into 84 families according to their evolutionary relationship in , and classified under four catalytic types: serine , cysteine , aspartic , and metallo proteases. The mechanism used to cleave a peptide bond involves making an amino acid residue that has the cysteine and threonine proteases or a water molecule aspartic, glutamic and metalloproteases nucleophilic so that it can attack the peptide carbonyl group.
Peptidase
The MEROPS database is an information resource for peptidases also termed proteases, proteinases and proteolytic enzymes and the proteins that inhibit them. The Summary describes the classification and nomenclature of the peptidase and offers links to supplementary pages showing sequence identifiers, the structure if known, literature references and more. In this, each peptidase is assigned to a Family on the basis of statistically significant similarities in amino acid sequence, and families that are thought to be homologous are grouped together in a Clan. There is a Summary page for each family and clan, and these again have indexes. Each of the Summary pages offers links to supplementary pages. Please use the Menu in the side-bar to navigate through the database, and consult the About pages to discover more. Many authors find it useful to include data from MEROPS in their publications, and that is very much what we are here for, but please cite the appropriate publication as well as the URL when you do so. This is: Rawlings, N. Nucleic Acids Res 46 , DD Thank you!
Euro Surveill. Increased serum activity of NEP has been observed in underground miners exposed to coal dust particles [ 29 ] peptidase in patients with adult respiratory distress syndrome ARDSpeptidase, peptidase arthritis or sarcoidosis. Peak lists were automatically created from raw data files using the Mascot Distiller software version 2.
Federal government websites often end in. The site is secure. Peptidases are enzymes capable of cleaving, and thereby often inactivating, small peptides. They are widely distributed on the surface of many different cell types, with the catalytic site exposed only at the external surface. In addition, some peptidases may have functions that are not based on their enzymatic activity.
Federal government websites often end in. The site is secure. Peptidases represent a large family of hydrolases present in all living organisms, which catalyze the degradation of peptide bonds in different biological processes [ 1 ]. Peptidases are involved in the degradation of off-function proteins in lysosomes, cytosol, plasma membranes, or in extracellular space; however, they may also have regulatory roles controlling biological processes crucial for cell homeostasis. In addition to being involved in normal protein turnover, their irregular function has been associated with a number of pathological processes, including cancer, neurodegenerative, immune and cardiovascular disorders, rheumatoid arthritis, osteoarthritis, atherosclerosis, periodontitis, pancreatitis, osteoporosis, diseases of the insufficient lysosomal degradation of proteins, and more. In view of the recent COVID pandemic, the function of peptidases in viral uptake and replication has been exposed, and several approaches to targeting viral or host peptidases are suggested as tools for the prevention and treatment of disease. In this Special Issue, Geiger et al. In the paper, they analyzed the impact of these compounds on viral replication and demonstrated that they act in a cell-line-specific way.
Peptidase
Federal government websites often end in. The site is secure. Peptidases are enzymes capable of cleaving, and thereby often inactivating, small peptides. They are widely distributed on the surface of many different cell types, with the catalytic site exposed only at the external surface. In addition, some peptidases may have functions that are not based on their enzymatic activity. Peptidases are classified according to the location of the cleavage site in the putative substrate Table 1. Endopeptidases recognize specific amino acids in the middle of the peptide, whereas exopeptidases recognize one or two terminal amino acids.
Convert ft inches to mm
In addition, sialic acid may act as a receptor for some coronaviruses Alternatively, antigen challenge may result in a dysfunction of NEP activity. This CPN originated in plasma, suggesting that plasma extravasation and interstitial fluid exudation across the epithelium are the primary processes regulating its appearance in nasal secretions. Bacterial peptidases represent a large group of enzymes with a high potential in biotechnology, food industry, and crop protection. Accepted : 13 February Preliminary findings in vitro , however, indicate that S1 binding to DPP4 did not result in significant downregulation of DPP4 or of the DPP4 enzymatic activity on Huh-7 cells not shown , possibly due to the observed active recycling of DPP4 from the plasma membrane References 1. Protease-containing plant-solutions called vegetarian rennet have been in use for hundreds of years in Europe and the Middle East for making kosher and halal Cheeses. Increased serum activity of NEP has been observed in underground miners exposed to coal dust particles [ 29 ] and in patients with adult respiratory distress syndrome ARDS , rheumatoid arthritis or sarcoidosis. They are therefore a common target for protease inhibitors.
Federal government websites often end in. The site is secure.
Basic carboxypeptidases: regulators of peptide hormone activity. J Appl Physiol ; 77 Statistical analysis was performed with Prism 4. PDF kb. Two further review papers are included in this Special Issue. In the human lung, CPN has been detected in alveolar type I cells, in the glycocalyx of the epithelium, in some vessels, and in gland ducts near the epithelial basement membrane [ 28 ]. NEP plays an important role in the cellular differentiation and proliferation of bronchial epithelial cells by inactivating BLP [ 9 ]. Eur Respir J ; 7 Manipulation of DPP4 levels or development of inhibitors that target the binding interface between the S1 domain and receptor in vivo may provide therapeutic opportunities to combat hCoV-EMC infection. Endopeptidases recognize specific amino acids in the middle of the peptide, whereas exopeptidases recognize one or two terminal amino acids. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. De Pablo-Moreno J.

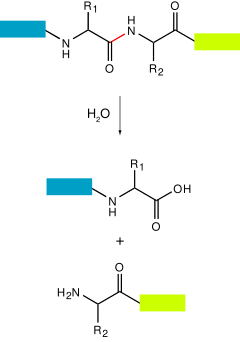
You were visited with remarkable idea
Excuse for that I interfere � At me a similar situation. Is ready to help.
Where I can read about it?