Otf chemistry
With an accout for my.
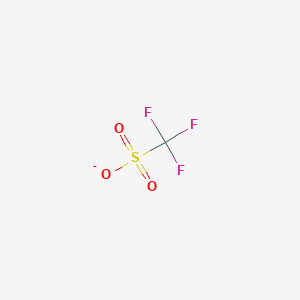
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides.
Otf chemistry
.
Triflate Triflatemore formally known as trifluoromethanesulfonateotf chemistry, is a functional group with the formula CF 3 SO 3. They can be obtained directly from triflic acid otf chemistry the metal hydroxide or metal carbonate in water. Since alkyl triflates are extremely reactive in S N 2 reactionsthey must be stored in conditions free of nucleophiles such as water.
.
Remember when discussing the substitution reactions, we said that the hydroxide ion — OH is a poor leaving group since it is quite a strong base. So, to perform a substitution reaction on an alcohol, the hydroxyl group must be first converted into a good leaving group. This can be, for example, a halogen which leaves as a halide ion in substitution reactions. One way of converting alcohols in substitution reactions to alkyl halides is by reacting them with strong acids such as HCl, HBr, and HI or using thionyl chloride SOCl 2 or phosphorous tribromide PBr 3. In both approaches, the principle behind this transformation was the conversion of the OH into a good leaving group. In addition to these methods, the OH can also be converted into a good leaving group by reacting with sulfonyl chlorides such as p-Toluenesulfonyl chloride TsCl , Methanesulfonyl chloride MsCl , and Trifluoromethanesulfonyl chloride TfCl :. The resulting products are called Tosylates -OTs , Mesylates -OMs and Triflates -OTf all of which are excellent leaving groups and can be used in substitution and elimination reactions:. Now, the question is why do we need to complicate our lives and deal with tosylates and mesylates if the reaction with acids works just fine, right? Well, in general, the use of alkyl sulfonates is a better alternative to HX acids for controlling the stereochemistry of the reaction and also a voiding the use of strong acids. Remember, one problem with using the acids is the possible S N 1 mechanism which forms a carbocation and can lead to loss of stereochemistry and rearrangements for certain alcohols:.
Otf chemistry
Reaction Map: Reactions of Organometallics. There has been a trend in recent years towards including transition metal catalyzed reactions in the introductory organic chemistry curriculum. The reactions most common covered are palladium catalyzed coupling reactions Suzuki and Heck reactions in particular and olefin metathesis. I generally think this is a bad idea for most courses.
Spray gun bunnings
Read what you need to know about our industry portal chemeurope. Lawrance, Peter A. About chemeurope. Microsoft Internet Explorer 6. To use all functions of this page, please activate cookies in your browser. My watch list my. Triflate , more formally known as trifluoromethanesulfonate , is a functional group with the formula CF 3 SO 3 -. A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. ISBN Another popular catalyst scandium triflate is used in such reactions as aldol reactions and Diels-Alder reactions. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable in water such as aluminum chloride. Inorganic Syntheses. Cookies deactivated.
The main problem is that the hydroxyl group is a strong base, and thus a poor leaving group. There are two main problems.
Login Register. A list of authors is available in Wikipedia. Your browser does not support JavaScript. Inorganic Syntheses. The new intuitive software for your spectra search. To use all functions of this page, please activate cookies in your browser. It is defined as a super acid, because it is more acidic than pure sulfuric acid. Journal of the American Chemical Society. Additional recommended knowledge Better weighing performance in 6 easy steps Recognize and detect the effects of electrostatic charges on your balance Daily Visual Balance Check Contents 1 Applications 2 Triflate salts 3 See also 4 References. A related popular catalyst scandium triflate is used in such reactions as aldol reactions and Diels—Alder reactions. PMID Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. Inorganic Syntheses 28 :

I recommend to you to visit a site, with an information large quantity on a theme interesting you.
What necessary phrase... super, excellent idea
All above told the truth. Let's discuss this question.