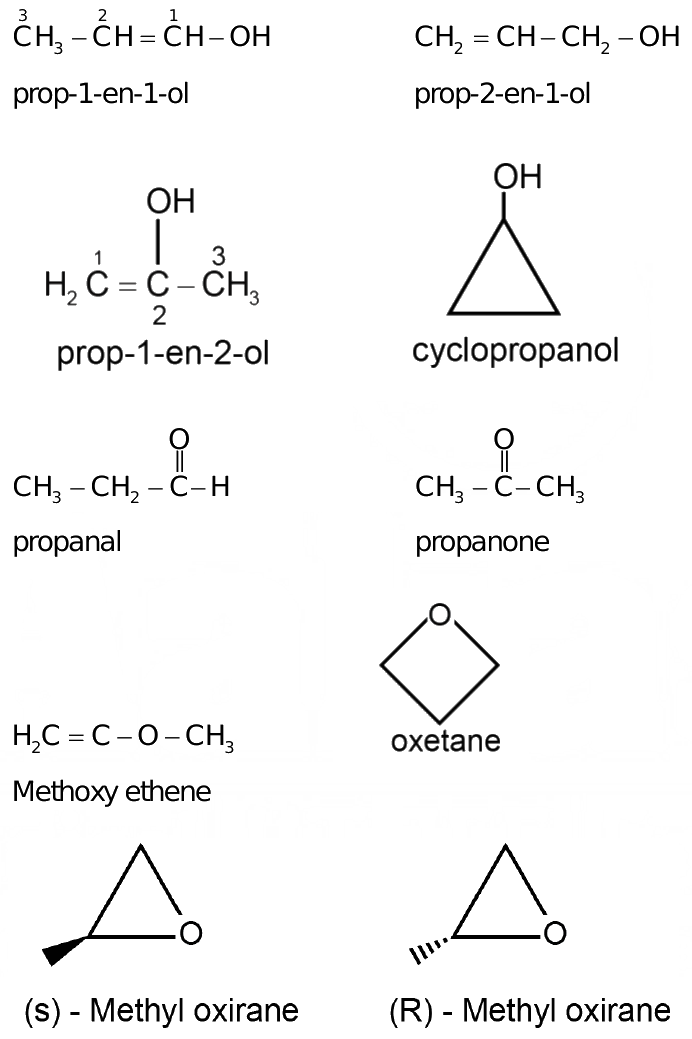
Number of structural isomers possible in c3h6o
Sign in Open App.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K.
Number of structural isomers possible in c3h6o
.
Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. View All Videos.
.
This page explains what structural isomerism is, and looks at some of the various ways that structural isomers can arise. Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers. Both are butane. There are also endless other possible ways that this molecule could twist itself. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
Number of structural isomers possible in c3h6o
Sign in Open App. Most Upvoted Answer. Prop1en1ol, 2. Prop1en2ol, 3.
Alterac swiss
Text Solution. In the given reactions, X and Y are :. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. Identify C in the following reaction: The different chemical formulas in structural isomers are caused either by a difference in what ligands are bonded to the central atoms or how the individual ligands are bonded to the central atoms. Signup now for free. The Best you need at One Place. The Question and answers have been prepared according to the Class 12 exam syllabus. Similar Class 12 Doubts Read the passage given below and answer the following questions:The existen Explore Class 12 courses.
After completing this section, you should be able to explain the differences among constitutional structural isomers and stereoisomers geometric isomers.
Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. Choose the most appropriate answer:Q. View more. Question Description No. Free Exam Preparation at your Fingertips! Create Account. In the given reactions, X and Y are : On reaction with HI, X gives a View All Tests. Prop1en2ol, 3. In the given reactions, X and Y are :. Similar Class 12 Doubts Read the passage given below and answer the following questions:The existence of coordination compounds with the same formula but different arrangements of the ligands was crucial in the development of coordination chemistry. Information about No.

It agree, this amusing message
Easier on turns!