Nitrogen trifluoride lewis structure
Nitrogen trifluoride NF3 is a colorless, nonflammable gas with routine usage in the microelectronics industry. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17, nitrogen trifluoride lewis structure, times that of carbon dioxide. The molecule is a hazardous greenhouse gas that can persist in the atmosphere for years.
The NF3 molecule, composed of one nitrogen atom and three fluorine atoms, holds within its structure a fascinating arrangement of atoms and electrons that govern its chemical behavior. By delving into the principles of valence electrons, formal charges, and the octet rule, we can decipher the molecular puzzle that NF3 presents. Determine Total Valence Electrons. Begin by identifying the number of valence electrons for each atom in the NF3 molecule. Nitrogen N is in Group 15 of the periodic table and has 5 valence electrons, while Fluorine F is in Group 17 and possesses 7 valence electrons. Choose the Central Atom.
Nitrogen trifluoride lewis structure
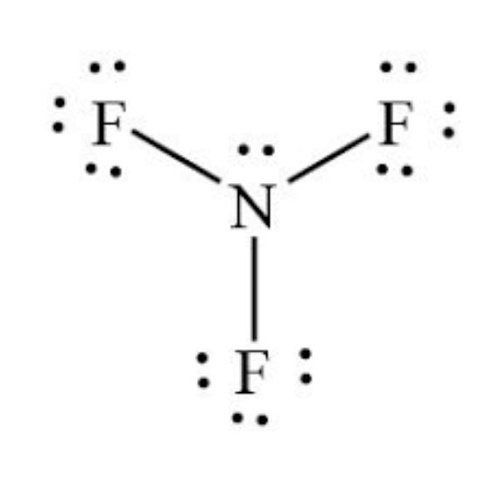
The Lewis structure of nitrogen trifluoride NF 3 consists of three N-F bonds and a lone pair on the nitrogen atom, while each fluorine atom has three lone pairs. Drawing the Lewis structure of NF 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. The Lewis structure of NF 3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs. In the Lewis structure of the NF 3 molecule, a central nitrogen atom is surrounded by three fluorine atoms. Each nitrogen — fluorine bond is represented by a single line, and there is a lone pair of electrons on the nitrogen atom. The three fluorine atoms are arranged in a trigonal pyramidal geometry such as ammonia molecule , with each fluorine atom positioned at a To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. Finally, the stability of the structure needs to be checked, and any charges on the atoms minimized by converting lone pairs to bonds to obtain the best Lewis structure. The total number of valence electrons in NF 3 can be determined by considering the two elements present: fluorine and nitrogen. Fluorine , a group VIIA element, has seven electrons in its valence shell, while nitrogen , a group VA element, has five electrons in its valence shell.
Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17, times that of carbon dioxide.
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule.
Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. One of the main reasons why this chemical compound is an essential topic is because it is a greenhouse gas. It is said to have quite high global warming potential but a comparatively low value of radiative forcing. Other than being a greenhouse gas and contributing to the climatic change of the planet, NF3 has several applications. It is used to produce chemical fluoride lasers and remove silicon-based compounds during semiconductor manufacturing.
Nitrogen trifluoride lewis structure
Transcript: Hi, this is Dr. We're going to do the Lewis structure for NF3, nitrogen trifluoride. On the periodic table, Nitrogen is in group 5 or 15, so it has 5 valence electrons; and then Fluorine is in group 7 or 17, it has 7. We've got three Fluorines, though, so let's multiply that by 3. That equals, 21 plus 5, is 26 valence electrons.
Time now in texas usa
Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. Determine Total Valence Electrons. So, we have successfully obtained the Lewis structure of NF 3. Mar 12, Connect the nitrogen N atom to each fluorine F atom with a single bond a pair of electrons. The molecule is a hazardous greenhouse gas that can persist in the atmosphere for years. Finally, the stability of the structure needs to be checked, and any charges on the atoms minimized by converting lone pairs to bonds to obtain the best Lewis structure. To evaluate the stability of the Lewis structure, check for any formal charges. The Lewis structure of NF 3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs. In the NF3 Lewis structure, lone pairs are placed on atoms to fulfill the octet rule and achieve electron stability. Each fluorine atom shares one electron with the nitrogen atom, resulting in three covalent bonds in the NF3 molecule. In the case of NF3, nitrogen N and fluorine F atoms share electrons to form covalent bonds. This is because nitrogen has five valence electrons, and it forms three covalent bonds with fluorine atoms, leaving one pair of electrons unshared or in a lone pair. Is it good for health? With two lone pairs and three bond pairs 6 electrons total , the central nitrogen N atom has a total of 8 valence electrons, satisfying the octet rule.
Nitrogen trifluoride NF 3 is an inorganic, colourless, non-flammable, toxic gas with a slightly musty odour. In the NF 3 molecule, nitrogen is attached to three fluorine atoms via a single bond and has a molecular weight of
With two lone pairs and three bond pairs 6 electrons total , the central nitrogen N atom has a total of 8 valence electrons, satisfying the octet rule. In the case of NF3, nitrogen N and fluorine F atoms share electrons to form covalent bonds. Facebook messenger. To evaluate the stability of the Lewis structure, check for any formal charges. Step by step drawing the Lewis structure of NF 3 To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. Begin by identifying the number of valence electrons for each atom in the NF3 molecule. Search for: Search Button. Fluorine is a group VIIA element and has seven electrons in its last shell valence shell. Therefore, we do not need to worry about reducing charges on atoms to get the best stable structure. Distribute the remaining valence electrons around the central nitrogen N atom as lone pairs, following the octet rule. Since there are already three N-F bonds in the molecule, there are ten 13 — 3 electron pairs left to be marked on the atoms. NF3 Lewis Structure.

It is remarkable, it is very valuable piece
Yes, really. So happens. We can communicate on this theme.
The useful message