N2o lewis dot
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion.
N2o lewis dot
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes.
Body Centered Cubic Unit Cell. A double bond between two atoms is shorter and stronger than a single bond between the same two atoms. Intro to Acid-Base Titration Curves.
Cronk Syllabus Topics. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot structures. These symbolic representations were introduced by Gilbert Newton Lewis , a prolific American chemist who was a pioneer in the theory of chemical bonding, thermodynamics, and other areas of chemistry. Lewis structures are of great utility as a tool that allows us to speak a "language" of chemical bonding and molecular structure.
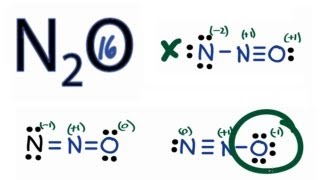
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table. Oxygen is a group 16 element on the periodic table.
N2o lewis dot
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons. Learn how to find: Nitrogen valence electrons and Oxygen valence electrons. We have a total of 16 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Italiannis menu
There are cases where even for very simple molecules there are several chemically plausible skeletal structures. One oxygen atom must have a double bond to carbon to complete the octet on the central atom. Here the choice will be made on the basis of a general rule - one we'll use again and explain further - that less electronegative atoms tend to be preferred as central atoms. What is a Lewis dot diagram? Gamma Emission. Solutions 2h 55m. Resonance Structures and Formal Charge. Titrations: Weak Acid-Strong Base. Ksp: Common Ion Effect. Aqueous Equilibrium 4h 42m. Complete Ionic Equations.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively.
Once we have a correct count of valence electrons, chosen a skeletal structure, and used pairs of electrons to make single bonds between the atoms of the latter, we'll need to place the rest of the valence electrons. Post by mbaker4E » Tue Nov 06, am. Polyatomic Ions. Henderson-Hasselbalch Equation. A few guidelines involving formal charge can be helpful in deciding which of the possible structures is most likely for a particular molecule or ion. Henry's Law Calculations. Octet rule Whenever possible, the valence electrons are distributed in such a way that eight electrons an octet of electrons surround each main-group element except hydrogen, which should have two electrons. Skeletal Formula. Intro to Acid-Base Titration Curves. Scientific Notation.

0 thoughts on “N2o lewis dot”