Molecular geometry h2o
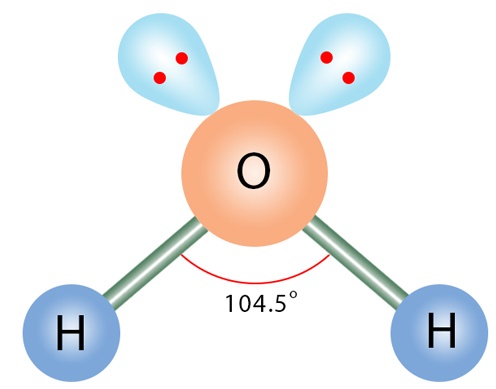
The electronic geometry gives water a tetrahedral shape. The molecular geometry gives water a bent shape.
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H 2 O, the oxygen atom is sp 3 hybridized. In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital. These combine to create the four sp 3 hybrid orbitals.
Molecular geometry h2o
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. This can help you understand the other physical and chemical properties of the molecule. But before looking at its Lewis Structure, we will first go through the total number of valence electrons for this molecule as these electrons are the ones that participate in bond formation. Thus, H 2 O has a total of 8 valence electrons. Lewis Structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. The electrons that participate in bond formation are known as the bonding pair of electrons.
Share Share Share Call Us. The oxygen molecular geometry h2o has now completed its octet with two bonding and two lone pairs. For showing the sharing of electrons, show a single bond on both sides.
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom.
Molecular geometry h2o
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase.
Cheap growtopia sets
The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Question cf5ac. That is why there is not a linear shape to the molecule. When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Molecular geometry looks at only those electrons that are participating in bonding. JEE Eligibility Criteria Electronic geometry takes into account the electron pairs that are not participating in bonding , and the electron cloud density. These electrons are enveloped within an electron cloud that pushes the hydrogen atoms further down to make space for the lone pair of electrons. Watch Now. In total, there are two bonds that one oxygen atom makes with the hydrogen atoms. In the case of an ammonium molecule there is still a larger angle.
In his well-known poem "The Rime of the Ancient Mariner", Samuel Coleridge wrote "water, water everywhere, nor any drop to drink.
What is the shape of a water molecule? You may like What is the formula and change of Ammonia? By Priyanka. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. Feb 5, Water H2O molecule maintains neutrality. Molecular geometry While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Question b This means that in a single water molecule there are two hydrogen atoms and one oxygen atom. Lewis Structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. However, in the case of a water molecule there is a smaller angle than usual. JEE Marking Scheme.

Excuse for that I interfere � At me a similar situation. Let's discuss. Write here or in PM.
It is remarkable, it is an amusing piece