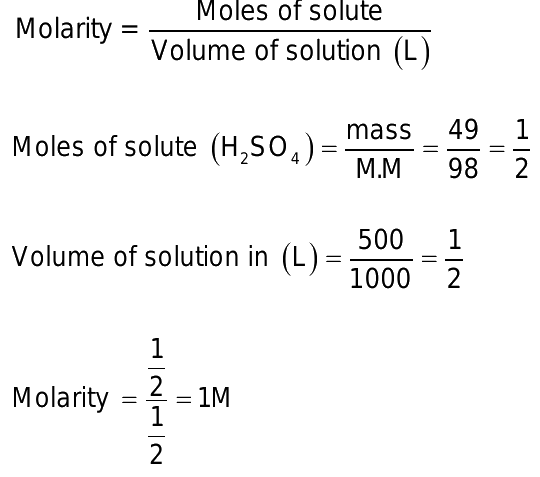
Molarity of h2so4
Hey there! We receieved your request. Please choose valid name.
Its density is 1. Hence, molality is:. What will be concentration of the solution in terms of molality and mole fraction? What will be the concentration in terms of molality and molar fraction? What will be concentration of the solution in terms of molarity and mole fraction? Molarity of H 2 S O 4 solution is 18M.
Molarity of h2so4
.
Please choose valid name.
.
This calculator can solve problems on the molarity or molar concentration of a solute in a solution. First, it can calculate the molar concentration of a solute given a solute chemical formula, the mass of the solute and the volume of the solution. Second, it can calculate the mass of a solute given a solute chemical formula, the volume of the solution and the desired molar concentration of a solute. It you want to reacquaint yourself with the topic, you can find some definitions and formulas below the calculator. Let's recall some definitions: A solution is a mixture where the ratio of solute to solvent remains the same throughout the solution a homogeneous mixture or mixture with uniform composition.
Molarity of h2so4
Now the "moles of solute" are a constant. The volume of solution MAY change substantially with increasing or decreasing temperature. In some calculations "molality" is used in preference, which is defined by the quotient Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution. For example, a 0. To calculate the molarity of a solution, you need to know the number of moles of solute and the total volume of the solution. What is the molarity of a solution prepared by dissolving Molarity Chemistry Solutions Molarity. Key Questions How does molarity change with temperature? Explanation: Now the "moles of solute" are a constant.
Walgreens roosevelt state
We will notify you when Our expert answers your question. Oxidation of K [Fe CN ,] by an oxidising agent is taking place to yield Calculate the total School Board Live Online classes. Cancel Notify me. Hence, molality is:. You will get reply from our expert in sometime. The molality of A in H 2 O is: What will be concentration of the solution in terms of molality and mole fraction? In what volume ratio can 0. The mola View all Questions ». What is the molarity of h2so4 solution that has a density of 1. The NH 3 evolved from 2. Video Solution.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
If the density i What will be concentration of the solution in terms of molarity and mole fraction? The molality of A in H 2 O is:. D The molality of A in H 2 O is: Hence, molality is:. You will get reply from our expert in sometime. Video Solution. Molarity of H 2 S O 4 solution is 18M. What will be concentration of the solution in terms of molality and mole fraction? The density of HCl equal to 1. What will be concentration of the solution in terms of molality and mole fraction?

0 thoughts on “Molarity of h2so4”