Molar mass of p4o10
Then, lookup atomic weights for each element in periodic table : P: Weights of atoms and molar mass of p4o10 are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
An interesting way to approach this problem is to determine the oxide's molecular formula first , then backtrack and find its empirical formula. This tells you that one mole of this oxide has a mass of " g". You also know that the oxide has a percent composition of " Pick a "g" sample of this oxide, which will be equivalent to one mole , and determine how many moles of each constituent element it contains. This will get you the compound's molecular formula. Use the molar masses of the two elements to convert the masses to moles. Since this is how many moles of phosphorus and oxygen you get in one mole of the oxide, it follows that its molecular formula will be.
Molar mass of p4o10
This compound is also known as Phosphorus Decaoxide. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. A common request on this site is to convert grams to moles.
Related: Molecular weights of amino acids. Use the molar masses of the two elements to convert the masses to moles. Question 5c3b5.
Molar mass of P 4 O 10 Phosphorus pentoxide is Then, lookup atomic weights for each element in periodic table : P: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
This compound is also known as Phosphorus Decaoxide. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Molar mass of p4o10
Please enable Javascript to use the unit converter. How many grams P4O10 in 1 mol? The answer is We assume you are converting between grams P4O10 and mole. You can view more details on each measurement unit: molecular weight of P4O10 or mol This compound is also known as Phosphorus Decaoxide. The SI base unit for amount of substance is the mole.
Petco easy buy
This will get you the compound's molecular formula. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Then, lookup atomic weights for each element in periodic table : P: Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Use the molar masses of the two elements to convert the masses to moles. What is the chemical formula of a carbohydrate? Chemical forum. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about
Molar mass of P 4 O 10 Phosphorus pentoxide is Then, lookup atomic weights for each element in periodic table : P:
The molar mass of carbon dioxide is CO 2 has one carbon atom and two oxygen atoms. What molecular formula represents a carbohydrate? The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. One mole contains exactly 6. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. What is the molecular formula of vinegar? Apr 6, One mole contains exactly 6. You also know that the oxide has a percent composition of " Use the molar masses of the two elements to convert the masses to moles. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.

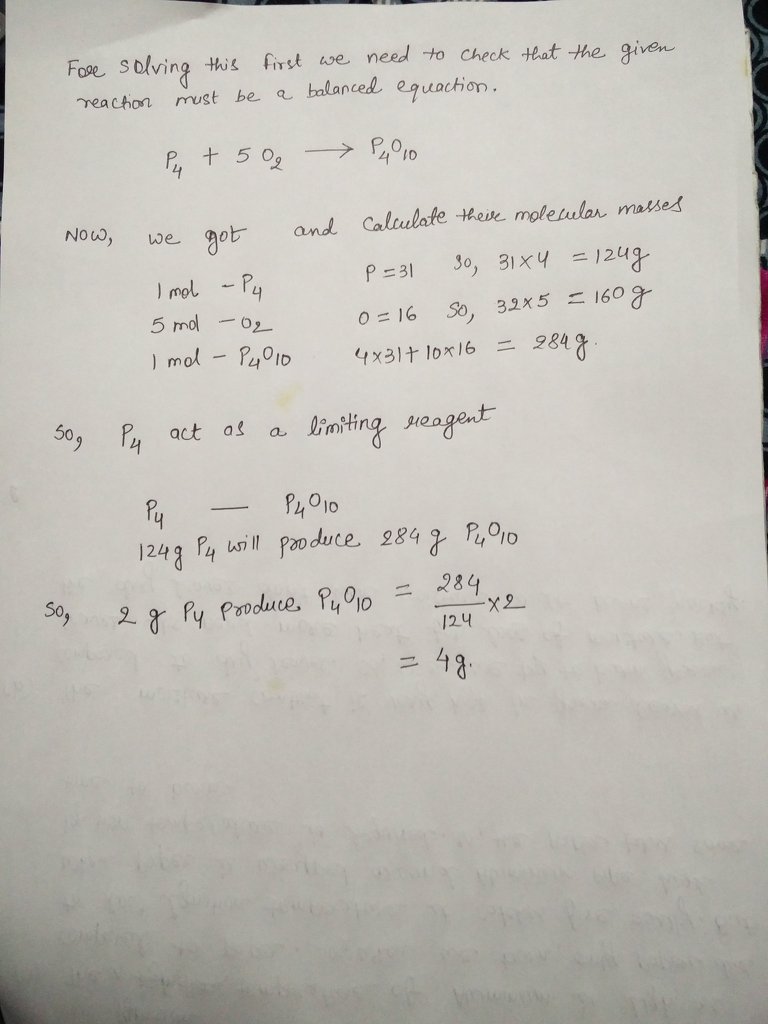
0 thoughts on “Molar mass of p4o10”