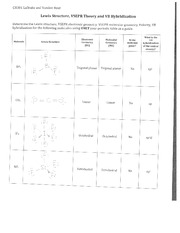
Lewis structures and vsepr worksheet answers
Log In Join. View Wish List View Cart.
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections. Documents Last activity. Add to
Lewis structures and vsepr worksheet answers
All of the resources on this site were written by Ian Guch email: misterguch chemfiesta. If I can give my hard work to others without cost, then you can do the same. By using these resources, you agree to do so at your own risk and hold Ian Guch blameless for anything bad that happens. Furthermore, you agree to use all prudent safety practices with your students esp. To be totally clear, you do NOT need to donate to use all aspects of this site and you never will. I ask that you consider donating to support the site, but a donation will never be required to use it. However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever. Just because some dumb guy on the Internet can make something blow up absolutely does not mean you should do the same. Leave that to the professionals. The Cavalcade o' Chemistry. Celebrating 25 years of chemistry goodness. Seriously, we've been around since !
Repulsions are minimized by placing the groups in the corners of a trigonal bipyramid. The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach.
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help. The spectrum shows that one of the electrons is different from the other three, and yet the four bonds of the carbon atom seem to be identical with one another I had the idea that the electrons might occupy four equivalent tetrahedral orbitals
Lewis structures and vsepr worksheet answers
Log In Join. View Wish List View Cart. Middle school. High school. Adult education.
Twizler
The Power Points contain the theory, the worksheets contain many practice pro. Suggest us how to improve StudyLib For complaints, use another form. Easel Activities. CHM Chapter 5 Document 29 pages. However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever. Add to collection s Add to saved. High school social studies. Search inside document. The central atom, carbon, has four valence electrons, and each oxygen atom has six valence electrons. Back to school. In molecular geometries that are highly symmetrical most notably tetrahedral and square planar, trigonal bipyramidal, and octahedral , individual bond dipole moments completely cancel, and there is no net dipole moment. High school.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
Chapter Five Document 46 pages. Place value. Thus a molecule such as H 2 O has a net dipole moment. Rate us 1. Is this content inappropriate? Miskon Stoi Document 21 pages. We also expect a deviation from ideal geometry because a lone pair of electrons occupies more space than a bonding pair. Any diatomic molecule with a polar covalent bond has a dipole moment, but in polyatomic molecules, the presence or absence of a net dipole moment depends on the structure. Add this document to collection s. This is a quick print and go activity - print out enough pages for your whole class, cut the pages down the middle, and you have a review activity that will keep them more engaged than a worksheet does. Groups are placed around the central atom in a way that produces a molecular structure with the lowest energy, that is, the one that minimizes repulsions. PreK social studies. All electron groups are bonding pairs BP , so the structure is designated as AX 3. Help and tutorials A. Modern Geometry of 00 Gall Rich Document pages.

I apologise, but, in my opinion, you commit an error. I suggest it to discuss. Write to me in PM, we will communicate.
What talented idea
You are not right. I can defend the position. Write to me in PM, we will discuss.