Lewis structure of sf6
This article is about the SF6 Lewis Structure, the Molecular geometry, and the formal charge present in the molecule. A Lewis Structure is a graphical representation of the valence shell electrons of a molecule.
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Draw the Lewis structure of C l O 4 per chlorate ion. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of nitric acid, H N O 3. Draw the Lewis structure for SF6. Which one of the following molecules contains no pi - bond?
Lewis structure of sf6
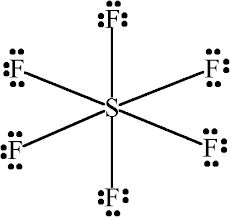
Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each fluorine atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around. The SF6 bond angle is 90 degrees. The SF6 Lewis structure is shown below:. Based on the information in the periodic table, we are able to obtain: Sulphur S and fluorine F are in the 16th and 17th group of the periodic table. The central atom must have a high valence or minimal electronegativity. For the SF6 molecule, sulfur has a maximum valence of 6 and fluorine has a maximum valence of 1; and Sulfur has a lower electronegativity than oxygen, so the sulfur atom is the central atom and the fluorine atom is the outer atom. For SF6 molecule, Total number of pairs of electrons are These lone electrons repel each other and maintain symmetry around the central atom. When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms e. Therefore, if possible, we should try to minimise the charges on the atoms. But in the SF6 molecule, we don't need to reduce the charge on the atoms because there are more than 8 electrons in the outer shell of sulphur, the reason is that some atoms can expand their valence shells to accommodate more electrons to achieve a stable structure. And the external fluorine atoms have formed an octet, so they are also stable.
A Lewis Structure is a graphical representation of the valence shell electrons of a molecule. Lewis structure of any molecule is important because.
.
SF 6 sulfur hexafluoride has one sulfur atom and six fluorine atoms. In the SF 6 Lewis structure, there are six single bonds around the sulfur atom, with six fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. In the periodic table , sulfur lies in group 16, and fluorine lies in group Hence, sulfur has six valence electrons and fluorine has seven valence electrons. Learn how to find: Sulfur valence electrons and Fluorine valence electrons. We have a total of 48 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Lewis structure of sf6
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts. Henri Moissan discovered the existence of SF6.
Best crop to start with fs22
The highest valence of fluorine is 1. Draw the Lewis structure of nitric acid, H N O 3. Some Other Uses of SF6. Order: 1g Purity: The dipole moment is cancelled due to the symmetric configuration. Lewis structure of any molecule is important because. In the SF6 molecule, the total number of electrons in the sulphur is 16, so the shell layer is populated with different energy levels depending on its capacity and level of hierarchy. Draw the Lewis structure of HCN. Even though sulfur only has four valence orbitals, the Lewis structure of sulfur hexafluoride specifies six pairs of electrons as bond pairs. The Polarity of Compounds. Which of the following is paramagnetic? As a result, arrange it in the middle and all Fluorine atoms around it. Sulfur hexafluoride Which one of the following molecules contains no pi - bond? With seven electrons in its outermost shell, fluorine is an element in group VIIA of the periodic table.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms.
Related Posts See All. View Solution. When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms e. Maximum valence: The maximum valence of Sulphur is 6. For ultrasonography, these microbubbles increase the visibility of blood vessels. Text Solution. The Lewis structure is important in chemistry because it can predict the number of bonds, nonbonding electrons, and bonding electron structure. Step 2 Identify the central atom The central atom must have a high valence or minimal electronegativity. In the SF6 molecule, the total number of electrons in the sulphur is 16, so the shell layer is populated with different energy levels depending on its capacity and level of hierarchy. This theory is concerned with electron repulsion and the need for compounds to take on a form to achieve stability.

In my opinion, it is an interesting question, I will take part in discussion. Together we can come to a right answer. I am assured.