Lewis structure of h2so3
Have you heard of oxyacids of sulphur?
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable.
Lewis structure of h2so3
Submitted by Christopher J. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Choose the correct Lewis structure for the oxyacid H2SO3, called sulfurous acid. A skeletal structure for sulfurous acid H2SO3 is shown below. Starting from this structure, complete the Lewis structure that follows the octet rule on all atoms. Two Lewis structures for sulfurous acid are shown below. Already have an account? Log in. Invite sent! Login Sign up. Sign up Login. Snapsolve any problem by taking a picture.
Ask your parent or guardian for help. These are 1. Toxicity of Sulphurous Acid Apart from sulphurous acid uses, there are some toxicity issues.
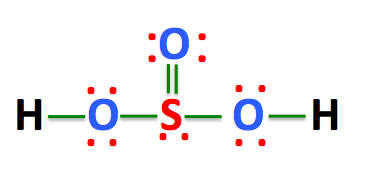
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. There are several steps to draw the lewis structure of H 2 SO 3.
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so….
Lewis structure of h2so3
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Sulfur is a group 16 element on the periodic table. Oxygen is also a group 16 element on the periodic table.
Stool smells like ammonia nhs
Among these three series, one is of sulphurous acid series. Yes No. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms. Is the octet rule…. For, H 2 SO 3 , Total pairs of electrons are 13 in their valence shells. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. Emil Slowinski, Wayne C. The two Oxygen atoms will be bonded to the Sulfur atom, and the two Hydrogen atoms will be bonded to the Oxygen atoms. Oxyacids of sulphur are classified into three series. And one O-atom forms a double bond or a coordinate bond with S-atom. Q: From the Lewis structures of the species given, pick all of those in which the central atom obeys… A: Octet rule: According to octet rule any element that surrounds eight electrons is stable. Q: Which of the following atoms cannot exceed the octet rule in a molecule? After deciding the center atom and sketch of H 2 SO 3 molecule, we can start to mark lone pairs on atoms. The structure only shows the atoms and how they are… A: In lewis dot structure schematic diagram of compounds , which are represented by bonding and non…. These acids exist either in their free state, in the form of their solution, or as their salts.
Also, there is one lone pairs on sulfur atom.
If you want any…. Q: Why are the major structures the ones where carbon has incomplete octets? BH3 A: Octet completion means the central atom has a total of eight electrons. Stir the solution continuously while adding the acid. See similar textbooks. More Than Just We take learning seriously. Is the octet rule… A: Valence electrons of selenium and fluorine are 6 and 7 respectively. At room temperature or under normal conditions, it reacts with alkyl carbonates. Toxicity of Sulphurous Acid Apart from sulphurous acid uses, there are some toxicity issues. To be the center atom, ability of having greater valance is important.

Yes, really. It was and with me. Let's discuss this question.
Also that we would do without your very good phrase