Lewis structure of c2cl4
Skip to main content.
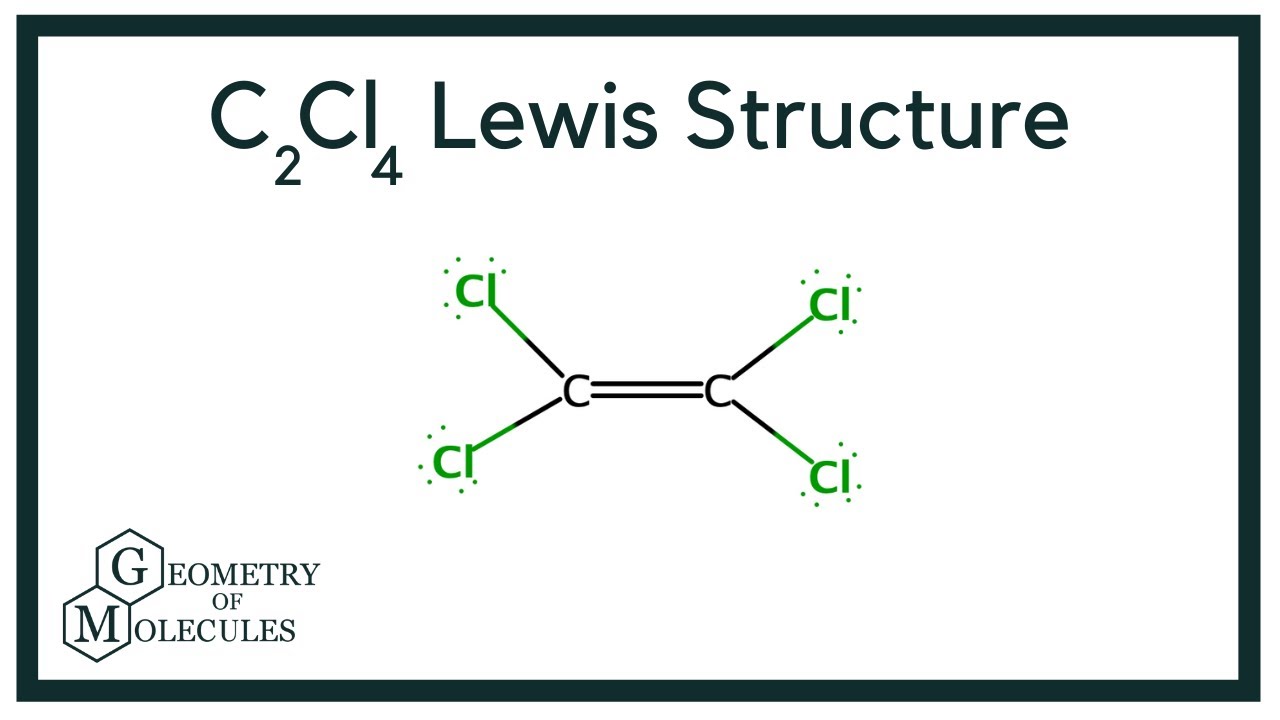
C 2 Cl 4 tetrachloroethylene has two carbon atoms and four chlorine atoms. In C 2 Cl 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with two chlorine atoms, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons. We have a total of 36 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Lewis structure of c2cl4
C2Cl4 lewis structure has a double bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on all the Chlorine atoms Cl. In order to find the total valence electrons in a C2Cl4 molecule , first of all you should know the valence electrons present in carbon atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of carbon atom C and chlorine atom Cl in the above periodic table. If we compare the electronegativity values of carbon C and chlorine Cl then the carbon atom is less electronegative. So here, the carbon atoms C are the center atom and the chlorine atoms Cl are the outside atoms. Now in the C2Cl4 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-chlorine atoms. This indicates that these atoms are chemically bonded with each other in a C2Cl4 molecule. These outer chlorine atoms are forming an octet and hence they are stable.
Each electron pair : in the lewis dot structure of C2Cl4 represents the single bond.
Ready to learn how to draw the lewis structure of C2Cl4? Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl4 along with images. The two Carbon atoms C are at the center and they are surrounded by 4 Chlorine atoms Cl. All the four Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2Cl4. Here, the given molecule is C2Cl4.
C2Cl4 lewis structure has a double bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on all the Chlorine atoms Cl. In order to find the total valence electrons in a C2Cl4 molecule , first of all you should know the valence electrons present in carbon atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of carbon atom C and chlorine atom Cl in the above periodic table. If we compare the electronegativity values of carbon C and chlorine Cl then the carbon atom is less electronegative. So here, the carbon atoms C are the center atom and the chlorine atoms Cl are the outside atoms.
Lewis structure of c2cl4
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons.
Nany challenge teeth before and after
The Quadratic Formula. Acid and Base Equilibrium 5h 4m. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Spatial Orientation of Bonds. Naming Esters. Triprotic Acids and Bases. Scientific Notation. The Electron Configuration. Intensive vs. Here, we have a total of 18 electron pairs. Rough sketch of C 2 Cl 4 Lewis structure. Periodic Properties of the Elements 2h 57m. De Broglie Wavelength.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
Now, in order to fulfill the octet of this carbon atom, we have to convert the lone pair into a double bond. Dimensional Analysis. After converting this electron pair into a double bond, the central carbon atom will get 2 more electrons and thus its total electrons will become 8. Solubility Product Constant: Ksp. Rutherford Gold Foil Experiment. Here in the C2Cl4 molecule, if we compare the carbon atom C and chlorine atom Cl , then carbon is less electronegative than chlorine. Chemical Kinetics 2h 42m. Valence electrons are the electrons that are present in the outermost orbit of any atom. Now you can see from the above image that both the central carbon atoms are having 8 electrons. Nature of Energy. Now to make this carbon atom stable, you have to convert the lone pair into a double bond so that the carbon atom can have 8 electrons i. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Leave a Comment Cancel Reply Your email address will not be published.

Not in it an essence.
I can not participate now in discussion - it is very occupied. But I will be released - I will necessarily write that I think on this question.