Lewis structure for clo2f
Q: A Lewis structure with placeholder elements is shown below, lewis structure for clo2f. If the formal charge of the central atom…. A: In the given Lewis structure, the central atom must have 5 valence electron which is possible for…. Q: A student proposes the following Lewis structure for the isocyanate NCo ion.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Chloryl fluoride molecule contains a total of 3 bond s. There are 3 non-H bond s , 2 multiple bond s , and 2 double bond s. Images of the chemical structure of Chloryl fluoride are given below:. The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Chloryl fluoride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
Lewis structure for clo2f
Chloryl fluoride is the chemical compound with the formula ClO 2 F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands. The related bromine compound bromyl fluoride BrO 2 F adopts the same structure as ClO 2 F, whereas iodyl fluoride IO 2 F forms a polymeric substance under standard conditions. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version. In other projects. Wikimedia Commons.
HArF ArF 2. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms.
Molar mass of ClO 2 F Chloryl fluoride is Then, lookup atomic weights for each element in periodic table : Cl: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as chlorite with the molecular formula ClO It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction. From this, it can be understood that chlorine dioxide in an ionic state will be a strong oxidizer of chlorine oxyanions. The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether. To begin drawing the Lewis structure of Chlorine dioxide, first, it is essential to draw one for the participating elements. Now, it will be easier to draw the Lewis structure of chlorine dioxide as we know that there are 20 valence electrons in one chlorine dioxide molecule.
Lewis structure for clo2f
We draw Lewis Structures to predict: -the shape of a molecule. For the ClO2- Lewis structure the total number of valence electrons found on the periodic table for the ClO2- molecule. Once we know how many valence electrons there are in ClO2- we can distribute them around the central atom with the goal of filling the outer shells of each atom. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron. To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets. It is helpful if you: Try to draw the ClO 2 - Lewis structure before watching the video. Watch the video and see if you missed any steps or information.
Cauliflower nutrition facts 100g
Include the formal charges on all atoms. Problem 27E: Without using Fig. ThF 4. Which resonance structure is best from a formal Images of the chemical structure of Chloryl fluoride are given below: 2-dimensional 2D chemical structure image of Chloryl fluoride 3-dimensional 3D chemical structure image of Chloryl fluoride. CoF 2 CoF 3. A: Lewis dot structure is the representation of molecule with its outermost electrons diagrammatically. A: Lewis structures are used to describe bonding pattern in simple molecules. Ball, Edward Mercer. Q: In developing the concept of electronegativity, Pauling usedthe term excess bond energy for the…. Problem E Problem E Problem E: Predict the molecular structure and bond angles for each molecule or ion in Exercises 87 and
Chloryl fluoride is the chemical compound with the formula ClO 2 F.
If the formal charge of the central atom… A:. ScF 3. Problem 35E: Predict the type of bond ionic, covalent, or polar covalent one would expect to form between the ThF 4. Since the question contains multiple sub-parts, the first three sub-parts shall only be…. Problem 28E: Without using Fig. Problem E: Write Lewis structures that obey the octet rule for the following species. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Organic Chemistry: A Guided Inquiry. A: To draw Lewis structure, the following steps are adopted,Calculate the total valence electrons…. InF 3.

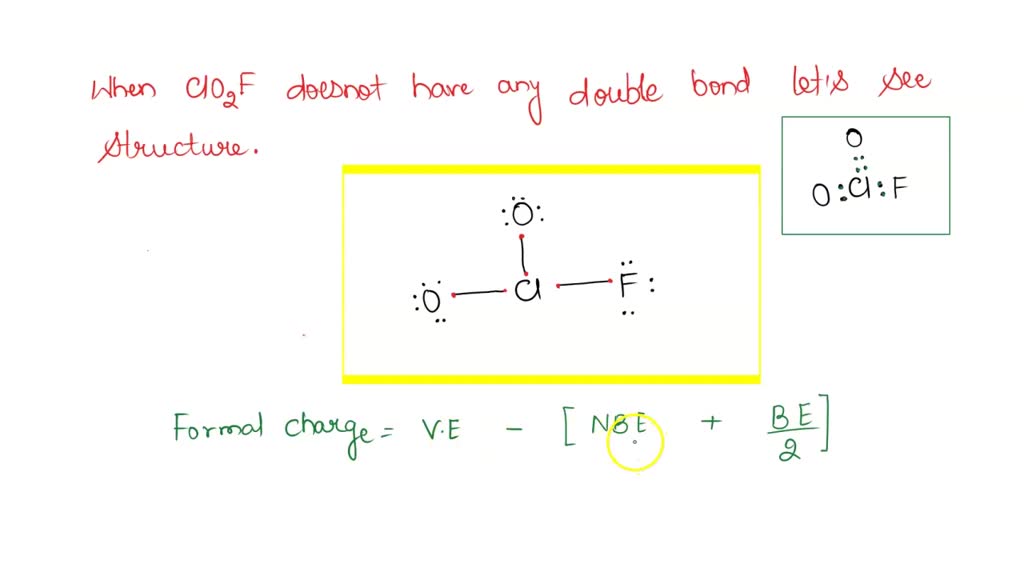
0 thoughts on “Lewis structure for clo2f”