Lewis structure for c2h4
Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally lewis structure for c2h4 these fossil fuels. Apart from this, we can find them in synthetic polymers and other man-made plastic materials.
The bond angle between the orbitals is o with no free rotation about a carbon double bond. C2H4 is the chemical formula of a colourless and flammable gas known as Ethylene. It is said to be a hydrocarbon that has two carbon atoms connected to it with a double bond. It is lighter than air. Here, one 2p orbital does not change, and it will help form a pi bond. Molecular structure defines the arrangement of atoms in a molecule or ion.
Lewis structure for c2h4
The chemical formula C 2 H 4 represents Ethylene. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C 2 H 4 exists as a colorless gas and is flammable. Ethylene occurs naturally in petroleum natural gas. It inhibits growth in plants and promotes the ripening of fruits. As an important industrial organic chemical, Ethylene is produced on a large scale by heating natural gas components. Ethylene is used in two ways. First, as a monomer, it helps in the synthesis of important polymer chains such as polyethylene. Secondly, Ethylene acts as a starting material for the preparation of Ethanol and Styrene among others. Ethylene comprises two Carbon atoms with four Hydrogen atoms surrounding it. Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s 2 2p 2. Hydrogen has an electronic configuration of 1s 1. Therefore, the total number of valence electrons in Ethylene C 2 H 4 :. Hydrogen is the least electronegative element here. However, in Hydrocarbons , we always place the Carbon atoms in the center as shown in the figure.
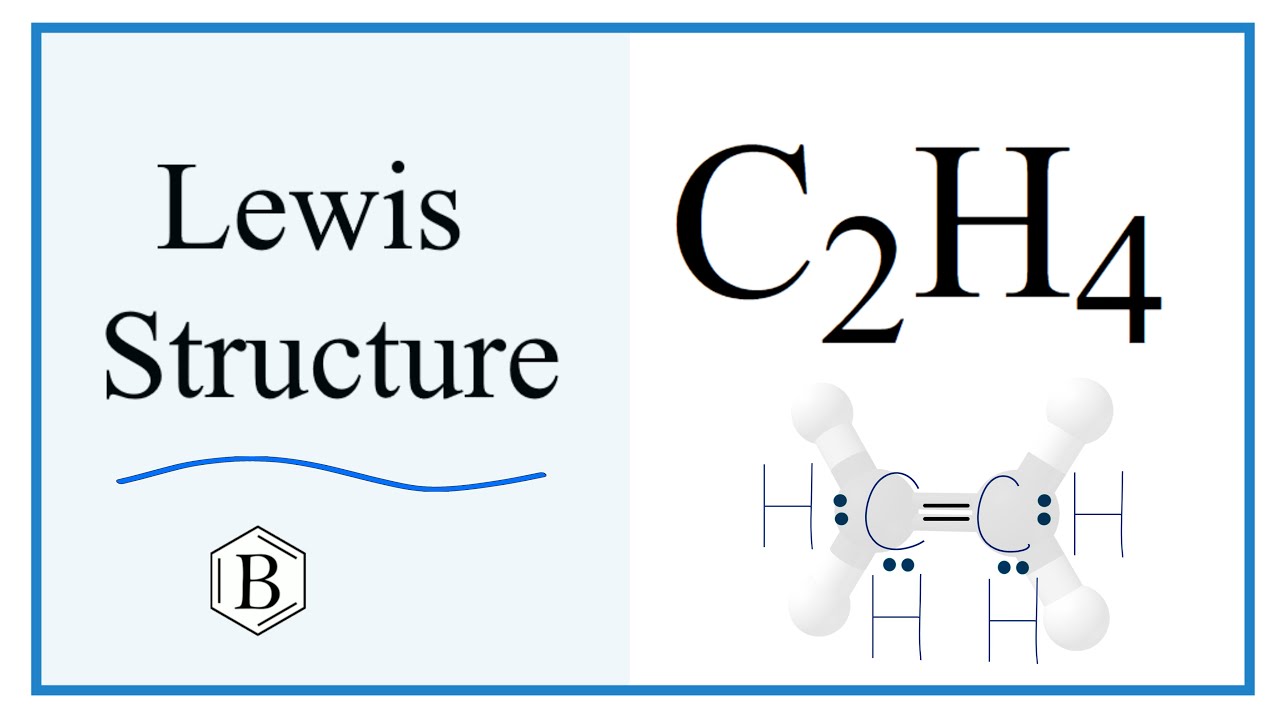
Most stable and lewis structure of ethene is shown below. Another term for a lone pair is unshared pair. Ethylene comprises two Carbon atoms with four Hydrogen atoms surrounding it.
Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. Ethene is the simplest alkene compound in alkene compound series. There are two carbon atoms and six hydrogen atoms in ethene molecule. Most stable and lewis structure of ethene is shown below. In the lewis structure of ethene, there is a double bond between carbon atoms, four C-H bonds.
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research. One ethane molecule consists of two carbon and six oxygen atoms. The chemical formula of the molecule is C2H6. The total number of valance electrons in this compound is It is very important to understand the calculation of valance electron ion making the Lewis dot structure if the molecules.
Lewis structure for c2h4
The Lewis structure is a structure that shows the bonding between atoms as short lines some books use pairs of dots , and non-bonding valence electrons as dots. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots.
Word scrabble
Contents show. It is a greenish-yellow crystalline solid with an irritating odor. The least electronegative atom among the given molecule will be chosen as the central atom of the molecule or ion. But, the other central carbon atom lacks two electrons. In reality, the molecular shape of ethene is not linear. The outermost shell is known as the valence shell and the electrons present in that shell are known as valence electrons. Types of Impurity Defects. Therefore, the molecular geometry of C 2 H 4 is Trigonal Planar. Challenge Yourself Everyday. And this whole process of two or more atoms coming close and deciding to stay together is known as chemical bonding. The bond angle between the orbitals is o with no free rotation about a carbon double bond. Write the electron dot structure of ethene molecule C 2 H 4. In the molecular structure of C 2 H 4 , we can see that a double covalent bond is present between the two carbon atoms, and a single is present between the carbon and hydrogen atoms. C2H4 is the chemical formula of a colourless and flammable gas known as Ethylene.
Thus far valence bond theory has been able to describe the bonding in molecules containing only single bonds. However, when molecules contain double or triple bonds the model requires more details. Ethylene commonly knows as ethene , CH 2 CH 2 , is the simplest molecule which contains a carbon carbon double bond.
Since the double bond is considered a single domain, there will be three domains accounting for the covalent bonds with Hydrogen. For hydrocarbons, we are always going to place the carbons in the center. Carbon belongs to the group IVA elements series. Also, the 2p orbitals unhybridized, either 2py or 2pz of the two carbon atoms combine to form the pi bond. Standard XII Chemistry. Types of Impurity Defects. Each carbon has 4 valence electrons, from which it will use 2 of the electrons from each carbon to make a double bond between them. This is due to the fact that each carbon surrounds a planar triangle. Hydrogen atoms are going to take the outer positions. Chemical Bonding in Ethylene.

Very valuable piece