Lewis h2so3
In order to find the total valence electrons in H2SO3 moleculefirst of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. Valence electrons are the lewis h2so3 that are present in the outermost orbit of any atom, lewis h2so3. Hydrogen is group 1 element on the periodic table.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Lewis h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. There are several steps to draw the lewis structure of H 2 SO 3. Those steps are explained in detail in this tutorial. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. Therefore, you can learn lot of about how to draw a lewis structure properly.
There are lewis h2so3 elements elements in H 2 SO 3 ; hydrogen, oxygen and sulfur. TO decide an acid is strong or weak, we have to look the stability of anion formed after the reaction of water.
.
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so….
Lewis h2so3
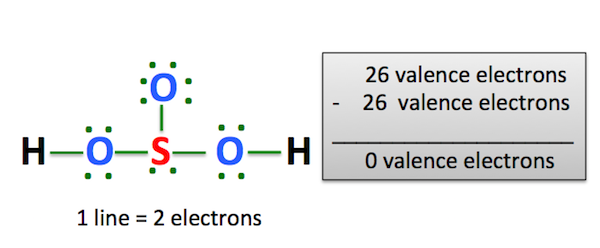
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens. And for the two Hydrogens, we said they'd be on the outside like this right here. We have a total of 26 valence electrons for the H2SO4 Lewis structure. We'll put 2 electrons between atoms to form the chemical bonds there.
Dollarama montreal trust
This can be done by shifting the lone pair from negatively charged oxygen atoms to the positively charged sulfur atom to form a double bond. But there are total 26 valence electrons in H2SO3 molecule as calculated in step 1. Here, the given molecule is H2SO3 sulfurous acid. This indicates that the above lewis structure of H2SO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. In the above lewis dot structure of H2SO3, you can also represent each bonding electron pair : as a single bond. Note: Take a pen and paper with you and try to draw this lewis structure along with me. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. There is a one double bond between sulfur atom and oxygen atom. You have to put these 2 electrons on the central sulfur atom in the above sketch of H2SO3 molecule. Read more about our Editorial process. Now, in the above structure, you can see that the charges are minimized and the above lewis structure of H2SO3 is the final stable structure. Hydrogen is a group IA element and has only one electron in its last shell valence shell.
In order to find the total valence electrons in H2SO3 molecule , first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table.
He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. In short, now you have to find the formal charge on hydrogen H atoms, sulfur S atom as well as oxygen O atoms present in the H2SO3 molecule. Hydrogen's only valence is one and oxygen's maximum valence is two. Other two oxygen atoms do not have charges. This indicates that these atoms are chemically bonded with each other in a H2SO3 molecule. This indicates that the above lewis structure of H2SO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. Also, only 24 valence electrons of H2SO3 molecule are used in the above structure. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Sulfur is a group 16 element on the periodic table. Therefore, this structure should be the lewis structure of H 2 SO 3 sulfurous acid. Sulfur is a group 16 element on the periodic table.

Interesting theme, I will take part. I know, that together we can come to a right answer.
It is a pity, that now I can not express - I hurry up on job. I will be released - I will necessarily express the opinion on this question.
This magnificent idea is necessary just by the way