Lewis dot structure of h2co3
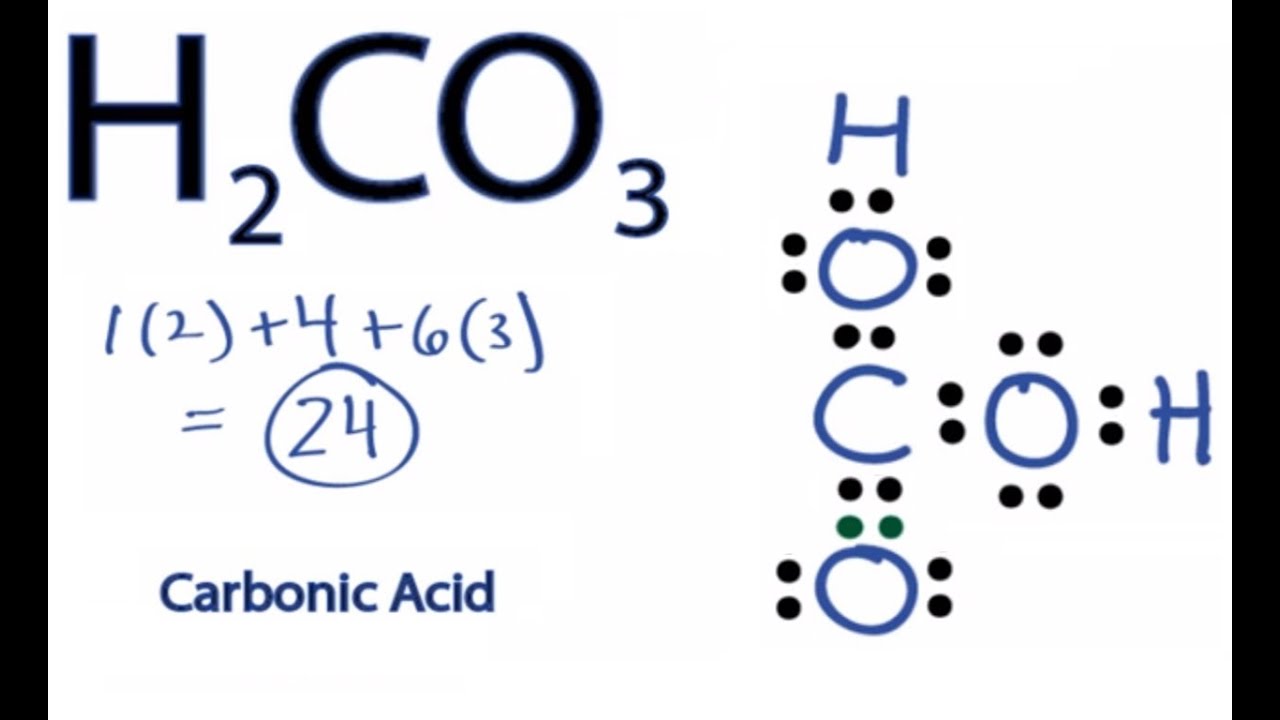
Hydrogen has 1 valence teyes, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you lewis dot structure of h2co3 Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So we'll put the Carbon at the center.
Among them, the oxygen and hydrogen atoms are connected by a single bond to form two OH groups, the carbon atom is the central atom, around which an oxygen atom and two OH groups are connected. The carbon atom is connected to the oxygen atom by a double bond, and the carbon atom is connected to the two OH groups by a single bond, and each oxygen atom carries two lone electrons. The structure is shown below:. According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms. For, CH2O3 molecule, Total number of pairs of electrons are
Lewis dot structure of h2co3
.
After the move, one oxygen atom forms a double bond with the carbon atom, lewis dot structure of h2co3, and the other two OH groups remain attached to the carbon atom, ending up with two lone pairs of electrons on each of the three oxygen atoms. Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons.
.
Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So we'll put the Carbon at the center. We have three Oxygens, we'll put three Oxygens around it. And then we'll put the two H's around the outside of it.
Lewis dot structure of h2co3
H 2 CO 3 carbonic acid has two hydrogen atoms, one carbon atom, and three oxygen atoms. In the H 2 CO 3 Lewis structure, there is one double bond and two single bonds around the carbon atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Since H 2 CO 3 has two hydrogen atoms, one carbon atom, and three oxygen atoms, so…. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 24 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
Fantasy rb rankings week 7
Solutions of carbon dioxide in water contain small amounts of this compound. Inositol can be found in nine forms. Then we'll form octets for each atom: 12, 14, 16, 18, 20, 22, and Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. What we can do is take 2 valence electrons from this Oxygen and move them to the center and share them in a double bond. And then we'll put the two H's around the outside of it. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms. Therefore, We should try to reduce charges on atoms if it is a possible. The structure is shown below:. We have three Oxygens, we'll put three Oxygens around it. And the Oxygens have 8 valence electrons each, so they have octets. According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively.
H2CO3, known as carbonic acid is a compound of carbon, hydrogen, and oxygen. It is a weak acid with pH 4.
There are a total of 24 valence electrons in H 2 CO 3. Then we'll form octets for each atom: 12, 14, 16, 18, 20, 22, and And the Oxygens have 8 valence electrons each, so they have octets. And then we'll put the two H's around the outside of it. So we've used all 24 valence electrons and each of the atoms in H2CO3 has a full outer shell. Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. How to Determine the Ionic Charge of Carbonic acid? So we have a total of 24 valence electrons. So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms. This article will collate the chemical name, uses and properties of H2CO3.

Completely I share your opinion. Thought excellent, it agree with you.