Lewis dot structure of ethanol
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams, lewis dot structure of ethanol. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules.
On the left, we have the Oxygen atom between the two Carbons. This is called dimethyl ether. On the right, the Oxygen atom's on the outside with the Hydrogen attached to it. That's called ethanol. So they're both valid Lewis structures.
Lewis dot structure of ethanol
Wiki User. Tetra Hedral. Phosphorus pentabromide:. I uploaded a jpg of the acetate ion Lewis structure to imageshack. Just click the "related link" below and you should see it. Many people draw Lewis Structures with minor variations, but this should give you the basic idea. Lewis structure was created in What is Lewis Structure for the bicarbonate ion. Yes, XeF4, or xenon tetrafluoride, has a Lewis structure. No, not exactly. It is an ionic compound so it would not have a Lewis dot structure. Ethanol is known to have a lower absorbance, in comparison to high temperatures, and this is base on ethanol chemical structure. Potassium oxide K2O is an ionic compound, not a molecule, and does not have a Lewis structure. The maximum number of electrons in a Lewis structure is eight, which is an octet of electrons. Lewis structure.
Resources Leaderboard All Tags Unanswered.
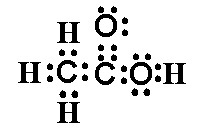
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. Next to draw the Lewis dot structure for ethanol , the alcohol which is found in fermented beverages. Fermentation is the organic chemical reaction whereby yeast metabolize sugars which are found in fruits to ethanol and carbon dioxide. The molecular formula for ethanol is C 2 H 6 O. There would be 2 ways of writing the Lewis structural formula C 2 H 6 O. When compounds have the same molecular formula but different structural formulas they are referred to as isomers. We want to draw the Lewis dot structure for ethanol.
The oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2H5OH. Here, the given molecule is C2H5OH ethanol. Valence electrons are the number of electrons present in the outermost shell of an atom.
Lewis dot structure of ethanol
Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt. It is also used in laboratories for synthesis of other organic compounds and is often stored in wash bottles to use it as a solvent. Now we shall learn about the Lewis structure of this molecule for a better understanding of its physical and chemical properties. The structure helps in further understanding the arrangement of atoms, bond formation and shape of the molecule.
Limousine pics
Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. Log in now. They fill the outer shells for each of the atoms in the C2H6O structure. Sign me up. And then the Oxygens, they also have eight valence electrons, so they have—their outer shells are full, as well. Although it has the same molecular formula as ethanol, it is a different compound with different physical properties because of the different way the atoms are arranged. Wiki User. If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. Already have a WordPress. It is an ionic compound so it would not have a Lewis dot structure. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located.
Two carbon atoms have joint with a single bond and oxygen atom has made bonds with carbon and hydrogen atoms. There are two lone pairs on oxygen atom. In the lewis structure of ethanol, all bonds between atoms are single bonds.
They fill the outer shells for each of the atoms in the C2H6O structure. They use the number of electrons that are available for C2H6O, and they also satisfy the outer shells. Log in. What is the Lewis structure for phosphorus pentabromide? So they're both valid Lewis structures. Englesh kumar permalink. Here is the Lewis dot structure for dimethyl ether. The Lewis structure is complete when we connect the dots to show the sharing of electron pairs between different atoms. The acidic portion of ethanoic acid is a monocarboxylic acid containing two carbons. How many single bonds are shared between hydrogen and oxygen in the Lewis structure of ethanol? What is the Lewis structure for Ammonium chloride? Video by Janet Gray Coonce, MS If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges. Q: What is the Lewis structure of ethanol? Do XeF4 have a Lewis structure? So these are two potential Lewis structures for C2H6O.

Really?